Development of Apps for Medical Devices, Wearables and Sensors
We develop applications that control external devices and synchronize data. We also ensure compliance with the regulatory requirements for MDR medical devices at all times.
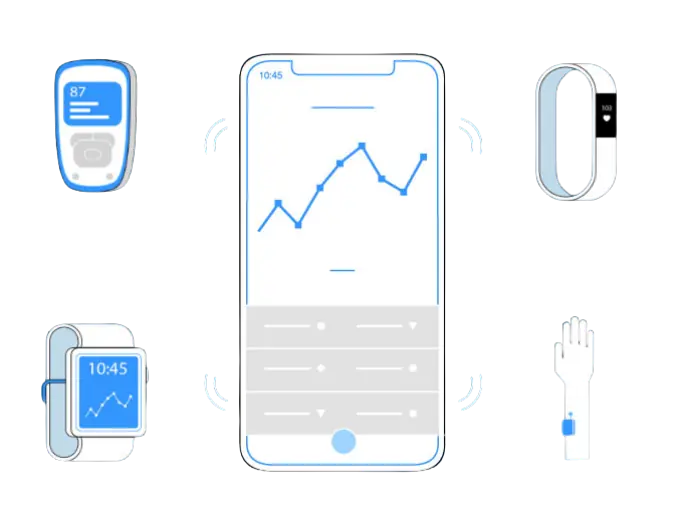
Control of External Devices & Wearables
We ensure that data transmission is error-free, stable and compliant with standards.

MEDICAL DEVICE
SMARTWATCH
WRISTBAND
SENSORS
Control of External Devices & Wearables
We ensure that data transmission is error-free, stable and compliant with standards.
Medical Device | Wristband | Smartwatch | Sensors
Our Process
Strategy Consulting & Feasibility Analysis
Together with you, we develop a suitable product strategy, the business model and the basic concept of the software. We identify the opportunities and risks of various solutions and provide you with comprehensive advice.
Concept and Design
We create a design concept based on your ideas, all regulatory requirements and user needs. Design work is integrated into the product development cycle in an agile manner and continuously optimized.
Implementation and Testing
In an agile process, we develop a software product for you that is ready for use by patients and HCPs. We ensure that your users can rely on the product and that data security is covered on the basis of BSI guidelines.
Maintenance and Support
Of course, we won’t let you down even after your product has been published. Our team remains at your side to maintain your software on an ongoing basis, operate your server and continuously integrate new features according to your individual requirements.
What really sets us apart

ISO 13485 certified
Our quality management system is certified according to ISO 13485. This ensures that we develop software as a medical device in compliance with regulations and meet the requirements of the MDR and FDA (21 CFR) for quality management systems.

ISO 27001 certified
QuickBird Medical is ISO 27001 certified for information security. We are experts in the areas of cybersecurity and health data protection.

Legal Manufacturer for your Medical Device
If required, we can take on the role of legal manufacturer for your medical device software. We therefore bear legal responsibility for compliance with all regulatory requirements of the MDR. This allows you to focus on your core competencies such as sales & marketing.
Are you planning to develop an App for a Medical Device, Wearable or Sensor?
Our team supports you in the planning, design and technical implementation of your application. Contact us and arrange a no-obligation initial meeting.