Development of Medical Software, Health Apps and DiGA
In the heart of Munich, we develop mobile apps, web applications and cloud systems for companies in the healthcare sector.
You are planning the implementation of a Medical Software or DiGA? In close collaboration with you, we take care of the conception, design and technical implementation of your software idea – certified according to ISO 13485 and ISO 27001.
Our Services
DIGITAL HEALTH APPLICATIONS
(DIGA)
We develop your DiGA in compliance with all regulatory requirements and support you on your way into the DiGA directory.
MEDICAL DEVICE SOFTWARE
(SAMD)
We develop your medical device software in accordance with the Medical Device Regulation (MDR) – certified in accordance with ISO 13485 and ISO 27001.
HEALTH SOFTWARE
& APPS
We develop your mobile app or web application that helps people to lead a healthier life in the long term.
LEGAL MANUFACTURER SERVICE
Certify a medical device without regulatory effort? We assume legal manufacturer responsibility for your medical device software or DiGA.
DECISION SUPPORT SYSTEMS
We develop customized software systems for your company that support specialists in the diagnosis or treatment of diseases.
FURTHER SERVICES
→ Wearables & Sensors
→ Reinforcement for your team
→ BSI TR-03161 for DiGA
– ePA & GesundheitsID
– Cybersecurity
– Telemedicine
– Artificial Intelligence
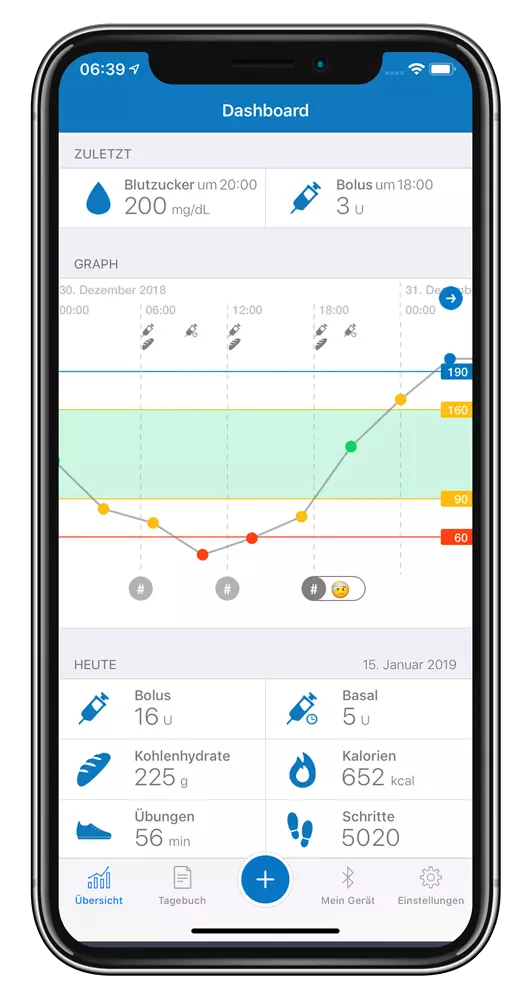
Neptun Diabetes Management
Medical apps that make life easier for patients
For medical technology companies and pharmaceutical companies such as Roche, we develop medical apps & software that make everyday life easier for patients. The app Neptun Diabetes Management automatically connects to the patient’s blood glucose meter and tracks measurements in the app. The patient no longer needs to take notes manually. They can also combine the data with their nutritional data to gain new insights.
TEDIRO
Software systems that relieve hospital staff
For companies like TEDIRO we develop software solutions that enable specialist staff in clinics to work more efficiently. The by QuickBird Medical developed Therapy Management System automates the planning, scheduling and implementation of pioneering gait training with robots.
Our Process
Strategy & Feasibility
Together with you, we develop a suitable product strategy, the business model and the basic concept of the software. We identify the opportunities and risks of various solutions and provide you with comprehensive advice.
Concept and Design
We create a design concept based on your ideas, all regulatory requirements and user needs. Design work is integrated into the product development cycle in an agile manner and continuously optimized.
Implementation and Testing
In an agile process, we develop a software product for you that is ready for use by patients and HCPs. We ensure that your users can rely on the product and that data security is covered on the basis of BSI guidelines.
Maintenance & Support
Of course, we won’t let you down even after your product has been published. Our team remains at your side to maintain your software on an ongoing basis, operate your server and continuously integrate new features according to your individual requirements.
Our drive
Whether we live a satisfied life depends on many factors. However, the foundation in any case is our physical and mental health. We want to contribute to the improvement of human health by developing medical software and health apps for medical technology companies, pharmaceutical companies and clinics. The common goal with our customers:
Enabling people to live healthier lives.

What makes us special

ISO 13485 certified
Our quality management system is certified according to ISO 13485. This means that we ensure compliant development of software as a medical device and meet the requirements of the MDR for quality management systems.

Data Protection and Information Security
QuickBird Medical is ISO 27001 certified for information security. We are experts in the areas of cybersecurity and health data protection.
You want to implement a medical software idea?
Contact us first without obligation. Together, we will take a look at the effort and time frame required for implementation. We can also provide technical feedback on possible procedures for developing the software.