DiGA Development 2024: Your Way into the BfArM Directory
Are you planning to develop a DiGA? Then arrange a non-binding consultation now. We will take care of software development, compliance with all regulatory requirements and support you on your way into the BfArM’s DiGA directory.
Our Roadmap for DiGA Development
The path to the DiGA directory is full of challenges. As a reliable partner, we accompany you from the idea to the software development to the listing of your DiGA in the directory of the BfArM.
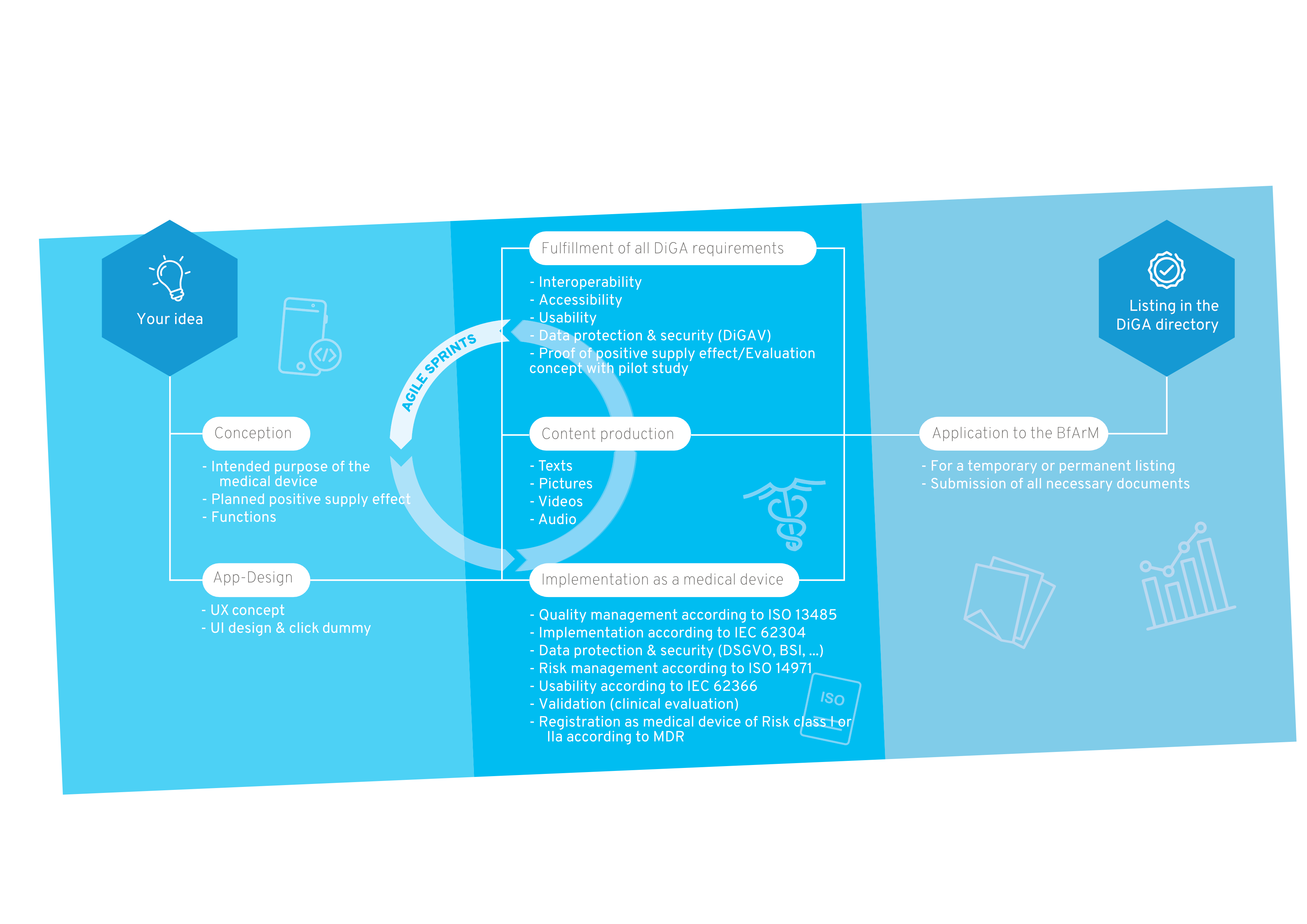
Your way into the DiGA directory
1. Conception
After the sparking idea, the conception begins, in which the target group, the benefit, the medical purpose and the requirements for your DiGA are defined.
UX and user research specialists

2. User Interface Design
A professional app design makes your app tangible for the first time and serves as an indispensable foundation for the implementation of the project. For visualization, we create wireframes and an initial click dummy, which is then filled with functionality.
3. MDR-compliant DiGA development
The regulatory jungle is obscure for many DiGA manufacturers at the beginning, because both the DiGAV and the MDR impose many requirements. In this step the functionalities of your DiGA are developed by us step by step, passing through our quality management processes according to ISO 13485 and IEC 62304. We are also familiar with the requirements in other countries, such as DiGA in France.

4. Content Production
A medical app requires good content. Once the scientific evidence for the content is given, it is translated into multimedia formats. We support you with the creation of images, videos, texts and other visual elements for a unique user experience.
5. Implementation as a medical device
Your app will then be registered as a Class I, IIa or IIb medical device according to MDR. We will guide you through this process and assist you in finding a designated body if necessary.

6. BfArM approval
We support you with the application to the BfArM and prepare all documents. After the app has been tested by the BfArM with regard to safety, functionality, quality, data security and data protection (fast-track procedure), it is provisionally reimbursed by the statutory health insurance for a period of one year.
7. Inclusion in the DiGA directory
Now your DiGA will be listed in the BfArM directory and can be reimbursed by health insurers. We take care of regular software updates and the maintenance of your product.
Additionally, we offer advice on marketing your DiGA to reach more patients.

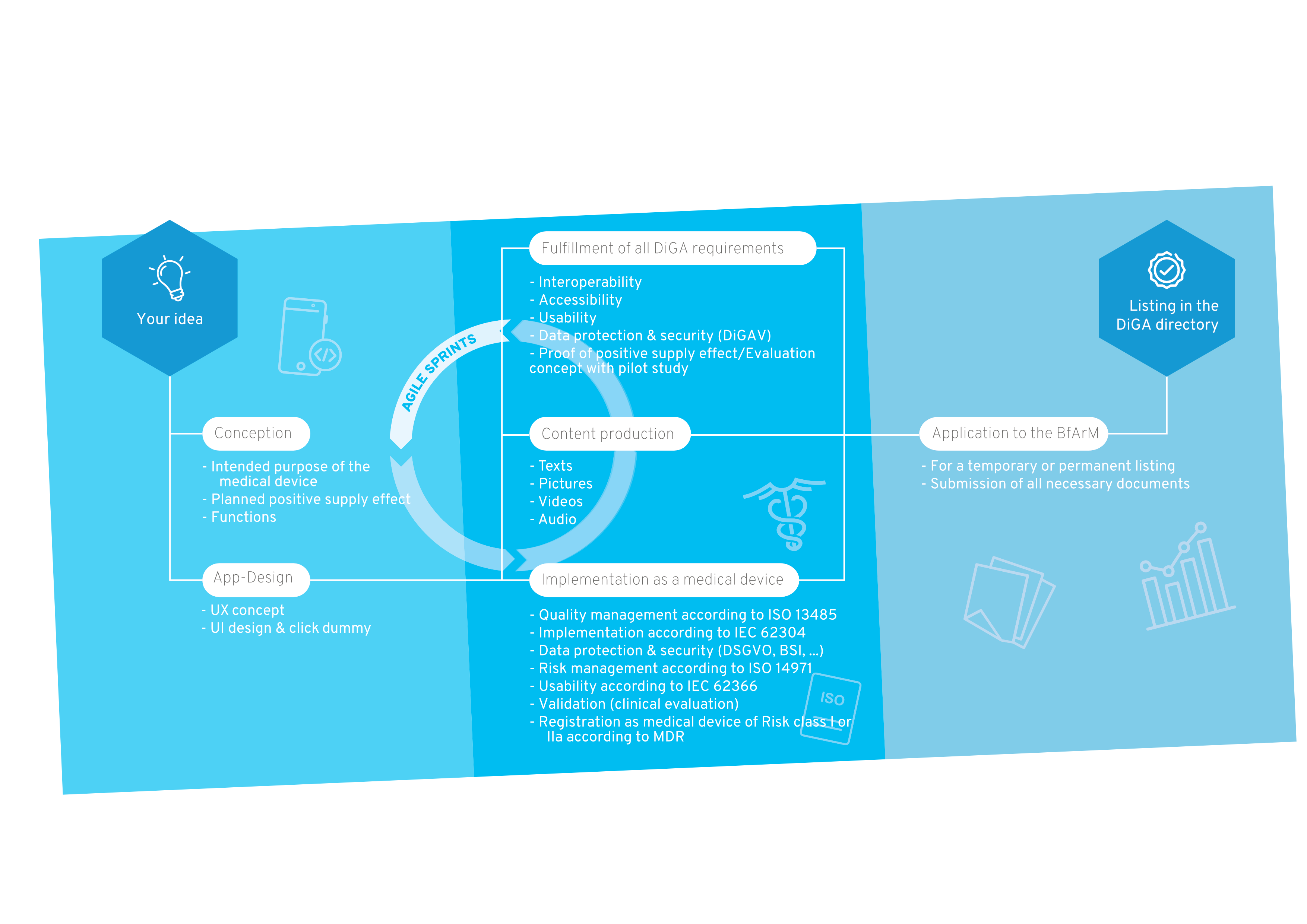
Your way into the DiGA directory

1. Conception
After the sparking idea, the conception begins, in which the target group, the benefit, the medical purpose and the requirements for your DiGA are defined.
UX and user research specialists

2. User Interface Design
A professional app design makes your app tangible for the first time and serves as an indispensable foundation for the implementation of the project. For visualization, we create wireframes and an initial click dummy, which is then filled with functionality.

3. MDR-compliant DiGA development
The regulatory jungle is obscure for many DiGA manufacturers at the beginning, because both the DiGAV and the MDR impose many requirements. In this step the functionalities of your DiGA are developed by us step by step, passing through our quality management processes according to ISO 13485 and IEC 62304.

4. Content Production
A medical app requires good content. Once the scientific evidence for the content is given, it is translated into multimedia formats. We support you with the creation of images, videos, texts and other visual elements for a unique user experience.

5. Implementation as a medical device
Your app will then be registered as a Class I, IIa or IIb medical device according to MDR. We will guide you through this process and assist you in finding a designated body if necessary.

6. BfArM approval
We support you with the application to the BfArM and prepare all documents. After the app has been tested by the BfArM with regard to safety, functionality, quality, data security and data protection (fast-track procedure), it is provisionally reimbursed by the statutory health insurance for a period of one year.

7. Inclusion in the DiGA directory
Now your DiGA will be listed in the BfArM directory and can be reimbursed by health insurers. We take care of regular software updates and the maintenance of your product.
Additionally, we offer advice on marketing your DiGA to reach more patients.
What distinguishes us in particular

ISO 13485 certified
Our quality management system is certified according to ISO 13485. In this way, we ensure a compliant development of your DiGA and fulfill the requirements of the MDR for quality management systems.

Data protection and information security
QuickBird Medical is ISO 27001 certified for information security. We are experts in the areas of cybersecurity and health data protection.

External placing on the market
If required, we can assume the role of legal manufacturer for your DiGA. We thus bear the legal responsibility for compliance with all regulatory requirements of the MDR. This allows you to focus on your core competencies such as sales & marketing.
Our Medical Software and DiGA customers
Are you planning to implement a DiGA?
Our team supports you in the planning and technical implementation of your app. In doing so, you benefit from our experience in implementing all regulatory requirements for DiGA. Contact us and arrange a no-obligation initial meeting. Let’s find out together how we can support you on your way to the DiGA directory.

