We will be the Legal Manufacturer for your Medical Device Software
Specialised Legal Manufacturer for Medical SoftwareWe assume manufacturer responsibility for your medical device software, medical app or DiGA. This includes ensuring overall regulatory conformity in accordance with MDR, DiGAV and ISO standards as the so-called legal manufacturer of the medical device.
We become the Legal Manufacturer for your Medical Device Software and/or DiGA
You are able to concentrate on your core competence and retain full control over your product. At the same time, we ensure compliance with the regulatory requirements for your MDR medical device. This means that you do not have to set up a quality management system (QMS) at your company and are not held back by regulations.

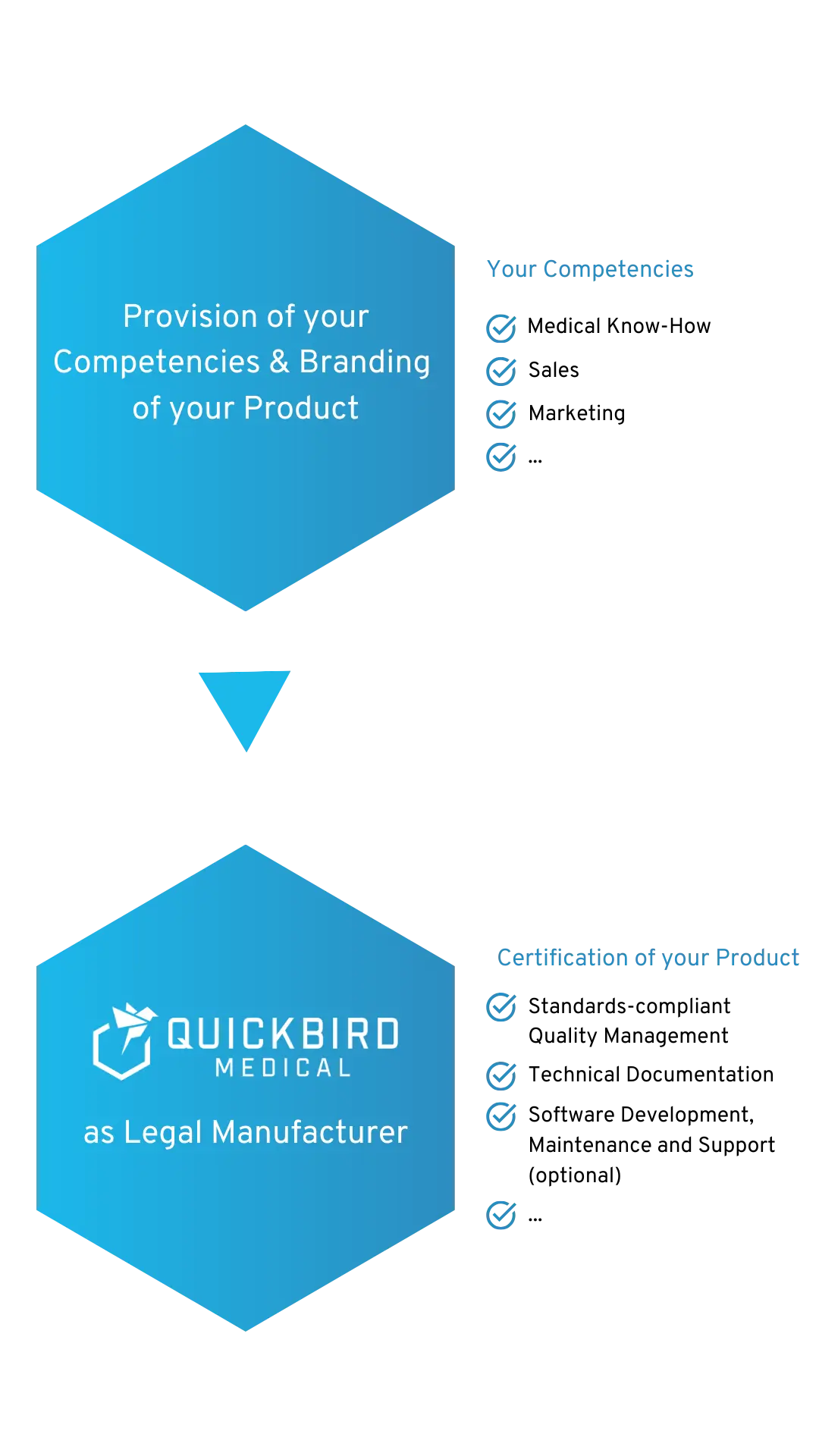
Certified according to ISO 13485 and ISO 27001

Specialized Expertise for Software Products

How we launch your product onto the market efficiently and safely. In the process we accompany and advise you on all relevant topics.
If required, we can also take over the (further) development, operation and technical support for your software.
Your Benefits
Maximum safety for your medical device

In-depth consultation for your SaMD

Faster market access

Concentration on core competence
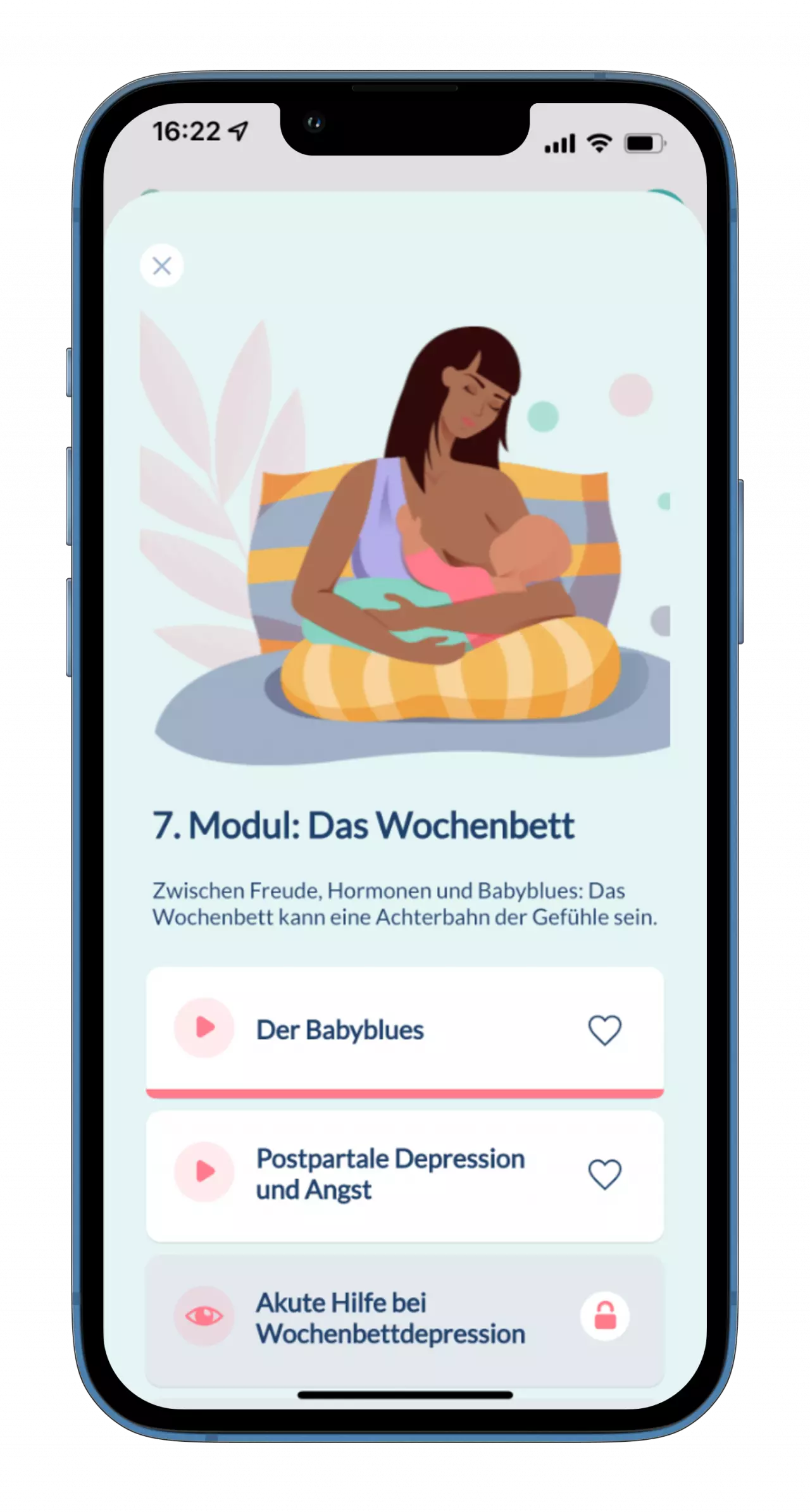
kontina
How we support Apogepha
as a Legal Manufacturer
kontina is a medical device for treating the symptoms of an overactive bladder (OAB). The app helps users to promote and improve their own bladder health in the long term. Read here how we are taking on the role of the Legal Manufacturer for this successful product.