BSI TR-03161 for DiGA – Implementation & Consulting
We help you to implement BSI TR-03161 within your digital health application (DiGA) quickly and securely. The common goal: to pass the BSI audit smoothly and obtain your BSI certification.
QuickBird Medical receives first BSI TR-03161 certification
QuickBird Medical has developed a DiGA (in application) in collaboration with a customer. After intensive preparation, careful implementation, and close cooperation with the testing center, the company achieved its goal on June 2, 2025: The application was one of the first DiGAs ever to receive the new BSI certificate in accordance with TR-03161. This is an important milestone for the entire DiGA scene.
QuickBird Medical receives first BSI TR-03161 certification
QuickBird Medical has developed a DiGA (in application) in collaboration with a customer. After intensive preparation, careful implementation, and close cooperation with the testing center, the company achieved its goal on June 2, 2025: The application was one of the first DiGAs ever to receive the new BSI certificate in accordance with TR-03161. This is an important milestone for the entire DiGA scene.
Our Expertise:
Implementation of BSI TR-03161 for DiGA
Our services include:

Gap Analysis
Identification of loopholes in your existing software product and processes.
Technical Implementation
Integration of BSI-compliant security requirements into your digital healthcare application and processes.
Preparations for Audits
We ensure that you are optimally prepared for the BSI audit. We also support you in preparing the necessary documentation.
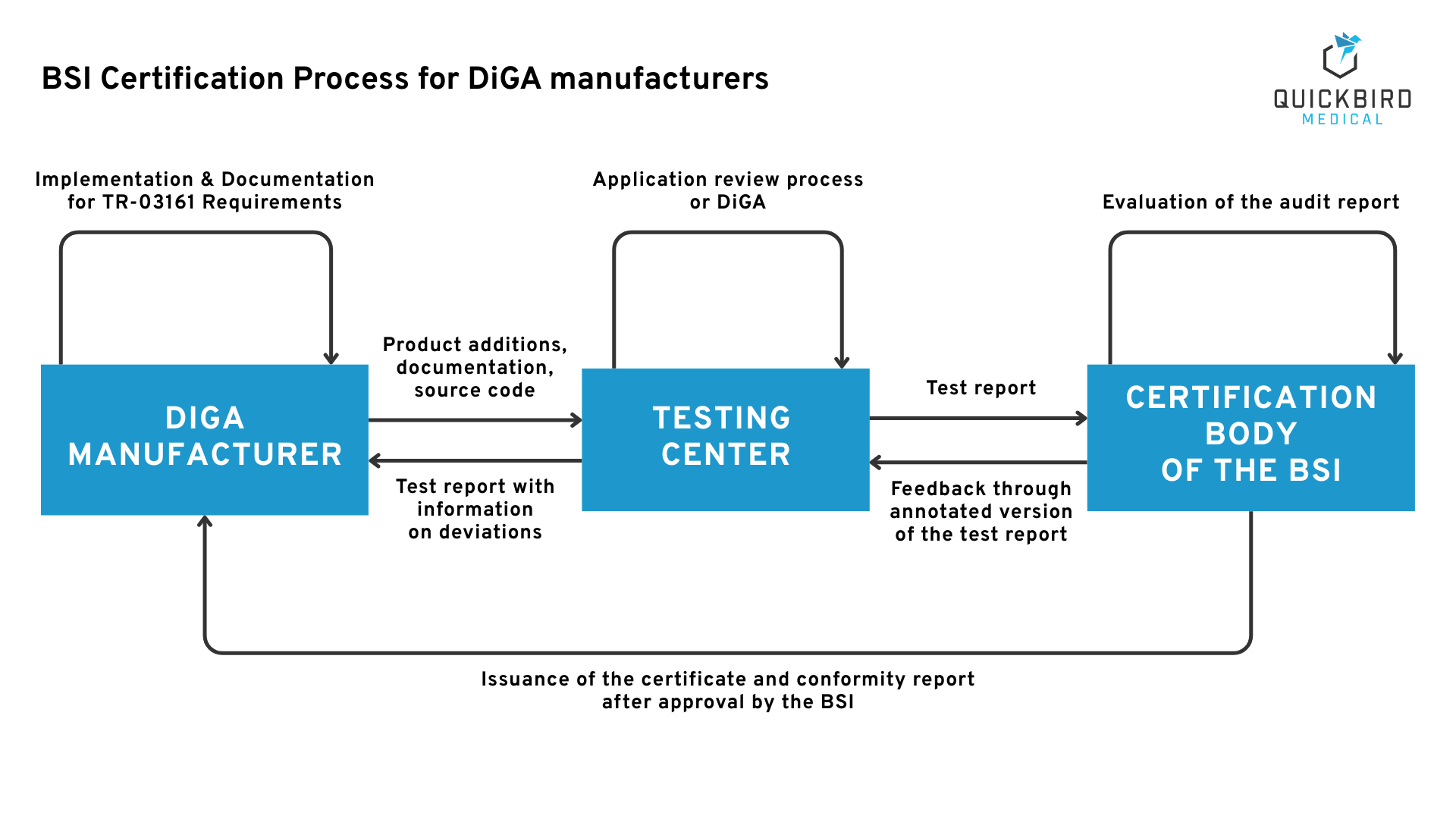
The BSI Certification Process
We are your partner for developing your DiGA, implementing BSI requirements and taking care of all other regulatory obligations. Thanks to our experience from over 15 DiGA projects, we are thoroughly familiar with the BSI certification process and can bring your DiGA to market safely.
How we work together
Initial Analysis
We evaluate your existing product and identify gaps that need to be closed before BSI certification.

Customized Implementation
Our software team implements all the necessary measures within your product or advises you on internal implementation.
BSI Audit Support
We prepare you comprehensively for the audit and support you in communicating with the inspection bodies.
What really sets us apart

ISO 13485 certified
Our quality management system is certified according to ISO 13485. In this way, we ensure the necessary quality of medical software.

ISO 27001 certified
QuickBird Medical is ISO 27001 certified for information security. We are experts in the areas of cybersecurity and health data protection.
What our customers say
Further information on BSI TR-03161

Practical guide to BSI TR-03161 for DiGA
Since January 1, 2025, manufacturers of digital health applications (DiGA) must prove compliance with data security requirements by means of an official certificate. The basis for certification is BSI TR-03161, which was first published in 2020. This guide covers all steps and aspects of the new data security certification.

Data security & data protection certificates for DiGA
Digital health applications (DiGA) require the following certificates in the area of data protection and data security (in addition to many other requirements) in order to be listed in the BfArM directory: the data security certificate according to BSI TR-03161 & the data protection certificate according to GDPR. We explain what the data protection and data security certificate is all about, when the certifications become mandatory, and how you can obtain them as a manufacturer.
Do you need BSI TR-03161 for your DiGA?
Contact us for a non-binding initial consultation. We will carry out an initial analysis of your existing product and accompany you on the way to successful BSI certification.