The new Digital Act of the Federal Ministry of Health was passed on February 2, 2024. It touches on a wide range of topics relating to modern healthcare, such as the electronic patient file (ePA) and regulations on the telematics infrastructure. With regard to DiGA, the Ministry of Health clarifies important points on how these digital solutions will be integrated into healthcare. For manufacturers, these changes mean both new opportunities and additional requirements for the approval of DiGA, which must be taken into account. We would like to present these to you below.
Overview
- 1. The Digital Act: DigiG – background and objectives
- 2. What are the new features for DiGA manufacturers?
- 2.1 Inclusion of risk class IIb DiGA
- 2.2 Phrasing of the pension entitlement of pregnant women
- 2.3 Pricing based on performance measurements
- 2.4 No 14-day return period
- 2.5 Sending of activation codes within 2 days by the health insurance companies
- 2.6 Interoperability
- 2.7 Telemedical monitoring
- 2.8 Innovation fund
- 2.9 Structured treatment programs for diabetes
- 2.10 Writing the DiGA in the ePA and implementing the health ID
- 2.11 Introduction and further development of the e-prescription
- 3. The DigiG’s path through the decision-making bodies
- 4. The opinions of the interest groups on the DigiG draft
- 5. Conclusion
1. The Digital Act: DigiG – background and objectives
The Digital Act (DigiG) is the law to accelerate digitalization in the healthcare sector. It was finally passed by the Federal Council in February 2024 in order to exploit the numerous potentials of the digital transformation in the healthcare and nursing sector. The DigiG aims to make the healthcare system more efficient, patient-centered, safer and of higher quality and to drive progress in care. It addresses the challenges associated with the introduction of digital solutions in the healthcare sector. Among other things, the law aims to integrate the electronic patient file across the board by means of an opt-out solution and to further develop the e-prescription in a binding manner in order to improve patient safety and the quality of care.
2. What are the new features for DiGA manufacturers?
2.1 Inclusion of risk class IIb DiGA
DiGAs in risk classes I and IIa can already be prescribed as part of standard care since the Digital Care Act (DVG) was passed in December 2019. What is new is that according to the Digital Act, DiGAs in risk class IIb can also be approved. However, manufacturers cannot apply for provisional approval, but must submit the positive proof of supply directly when applying for inclusion in the DiGA directory. This means that a DiGA cannot be prescribed during the assessment of the positive treatment effect, as would otherwise be the case in the fast-track procedure (provisional listing). This results in longer periods of time for manufacturers, which must be bridged until sales are generated.
2.2 Phrasing of the pension entitlement of pregnant women
The DigiG now explicitly lists the care of pregnant women with DiGA. This was necessary as a regular pregnancy as an ICD-10 code does not in itself constitute an illness. Nevertheless, the SGB V regulates medical care for pregnancy, birth and the puerperium. The explicit listing of digital health applications in the wording of SGB V is now intended to clarify that statutory health insurance covers the provision of DiGA for health problems associated with pregnancy.
The new wording in SGB V is now planned:
In Section 24c sentence 1 number 2, the words “and aids” are replaced by the words “, aids and digital health applications”.
§ Section 24e is amended as follows: In sentence 1, the words “and assistive devices” are replaced by the words “assistive devices and digital health applications”. (Translated from German)
Further amendments concern the paragraphs on exemption from co-payment in the event of pregnancy. However, a more detailed explanation of the delimitation of the entitlement to care in the case of regular pregnancy is still pending, meaning that this inclusion in the law continues to offer room for interpretation. This is because it is still not possible to prescribe DiGA solely on the basis of pregnancy.
2.3 Pricing based on performance measurements
Note the mandatory introduction of an application-related performance measurement for all DiGA listed in the directory as of January 1, 2026. The results of this performance measurement must be continuously reported to the Federal Institute for Drugs and Medical Devices (BfArM) and published in the directory. The Federal Ministry of Health also provides for a 20% share of “success-based price components” in the DigiG.
According to the BMG’s considerations, conceivable success criteria would be, for example, how many hours patients use a DiGA on average per day and whether the application is discontinued, but no specific criteria are mentioned in the law. However, as neither the National Association of Statutory Health Insurance Funds nor the manufacturers consider the criteria proposed by the BMG to be useful, the mandatory introduction of performance-based price components has been set for January 1, 2026 in order to allow more practice-relevant criteria to be determined.
This new requirement poses an additional challenge for DiGA manufacturers when it comes to positioning their products profitably on the market. On the one hand, appropriate tracking must be implemented, and on the other, the issue of adherence becomes even more important.
2.4 No 14-day return period
The original draft bill provided for a 14-day return period: Patients were to be able to independently decide against using a DiGA for the first 14 days. During this period, use would have been free of charge. This procedure would have been unique in the field of therapies prescribed by doctors. Not least due to the SVDGV’s objection, this paragraph was removed from the draft bill.
2.5 Sending of activation codes within 2 days by the health insurance companies
In the past, patients sometimes had to wait a long time for the activation codes required to use a DiGA to be sent to them. The health insurance companies responsible for delivery are now obliged to send these activation codes within two days.
2.6 Interoperability
Interoperable information systems are crucial for high-quality healthcare, but due to the fragmentation of the German healthcare system and the diversity of information systems, there is a risk of quality and quantity losses in the exchange of treatment-relevant data. In order to achieve the interoperability goals, the DigiG decided to increase the binding nature of standards and profiles, which should lead to improved data availability, higher treatment quality and greater protection of the health and informational self-determination of insured persons.
For DiGA, the DigiG refers to the regulation under Section 374a SGB V, which will apply from mid-2024. This is intended to create greater openness between medical aids/implants and DiGA, which should give DiGA access to more data sources in the future. You can find out more about this in our blog article.
2.7 Telemedical monitoring
The Digital Act provides for telemedicine to become an integral part of healthcare. In particular, video consultations are to be used even more extensively and be easier to access. As a first step, the previous restriction on video consultations will be lifted. It could be interesting for DiGA manufacturers that comprehensive monitoring concepts, including those involving DiGA, will be eligible for reimbursement. It should also be possible to integrate the TI Messenger into DiGA. As this primarily involves the further expansion of existing structures, the future will show whether there are any points of contact for DiGA manufacturers.
Source: Handelsblatt article on the draft DigiG
2.8 Innovation fund
The innovation fund is intended to build bridges between areas of healthcare that have previously had difficulty interacting. It is financed from statutory health insurance funds. Originally, this fund was limited until 2024. The innovation fund is being consolidated and expanded in order to promote the development of innovative forms of healthcare and healthcare research. However, the current application rules do not open up any new funding opportunities for DiGA manufacturers. Product-related studies cannot be funded and a direct economic interest in the study results is an exclusion criterion.
Click here for the Innovation Fund website.
2.9 Structured treatment programs for diabetes
The DigiG provides for the Federal Joint Committee (G-BA) to introduce new structured treatment programs for insured persons with type I and type II diabetes mellitus that are based on digitalized care processes. By integrating previously separate data sources for patients and service providers, this measure enables a care process that makes targeted use of digital technologies and thus allows for more effective treatment. Point 13 of the proposed amendments to SGB V specifically mentions “digital health applications” as part of these digitalized care processes. This offers interesting points of contact for DiGA manufacturers.
2.10 Writing the DiGA in the ePA and implementing the health ID
By April 30, 2024, the health ID and the DiGA letter must be implemented in the electronic patient file (ePA). The DigiG refers here to Section 374a SGB V, which already contains the legal requirements for this obligation.
2.11 Introduction and further development of the e-prescription
Prescriptions must be issued electronically from January 1, 2024. However, the DigiG also sets out more far-reaching goals. For example, it should be possible to read the electronic health card via an NFC-enabled smartphone and thus also redeem e-prescriptions at online pharmacies. However, the specifications are currently still being agreed.
3. The DigiG’s path through the decision-making bodies
The BMG’s DigiG draft bill under Karl Lauterbach still had to overcome various hurdles before it was finally passed by the Bundestag. The various negotiating bodies, including the timetable, are shown in the following diagram. The law was finally passed on February 2, 2024; you can find the current version here.
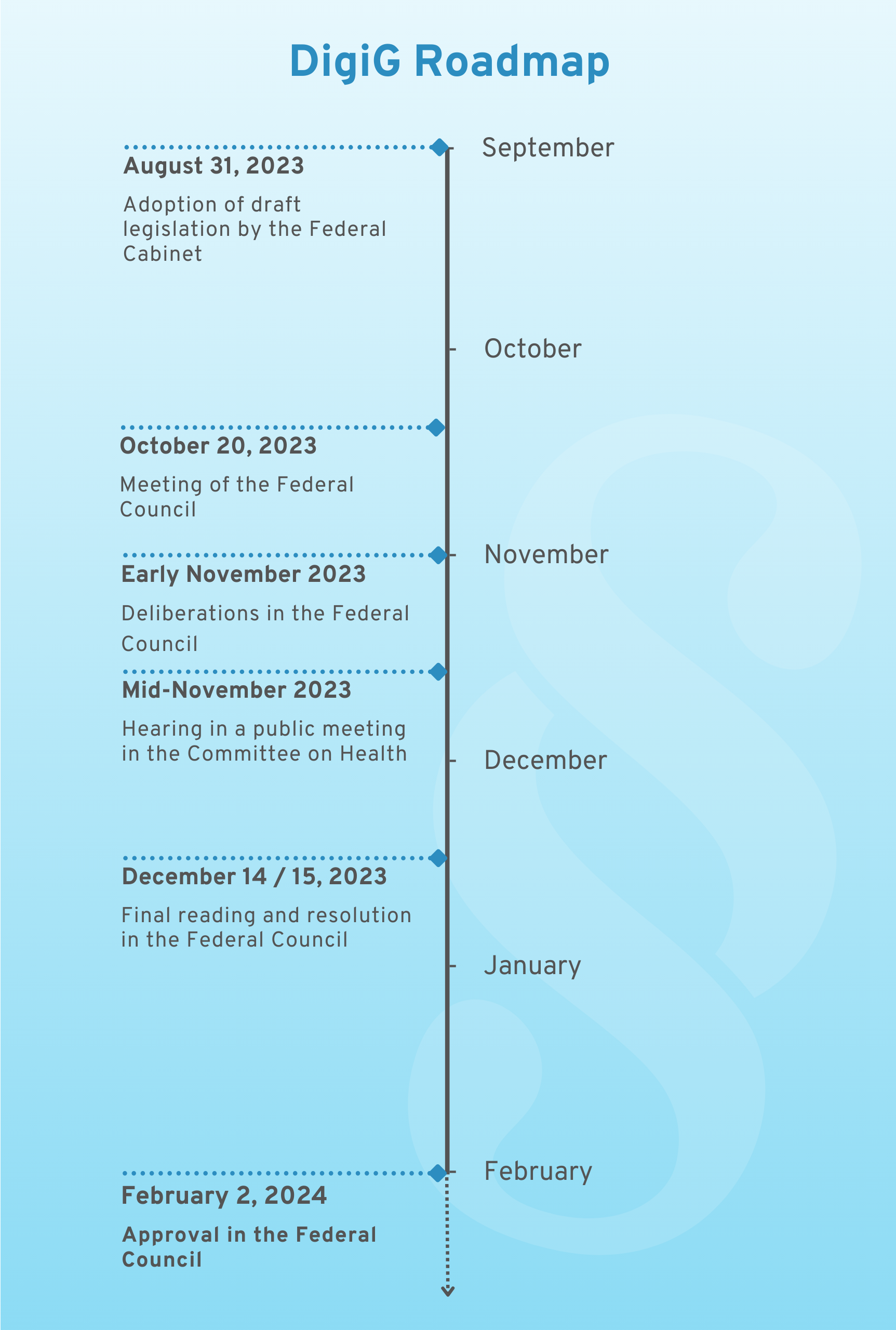
DigiG Roadmap
4. The opinions of the interest groups on the DigiG draft
In view of the intended introduction of the DigiG, both the health insurance companies and manufacturers submitted comprehensive comments on the draft bill. This resulted in significant adjustments to the final law. Below we explain the most important points of the comments made at the time:
4.1 Digital Healthcare Association (SVDGV) and German Medical Technology Association
The German Association for Digital Healthcare (SVDGV) has 169 members. These include many DiGA manufacturers. The possibility of bringing DiGAs in risk class IIb onto the market in future was welcomed. The SVDGV cannot understand why these DiGAs are excluded from the fast-track procedure according to DigiG. Numerous examples from the world of software could prove that a medical device in a higher risk class is not automatically more complex and dangerous. The German Medical Technology Association even sees the success of DiGA at risk as a result of this new requirement. The company representatives are calling for all DiGA manufacturers to be granted a trial phase.
The SVDGV also rejected the proposal in the DigiG, which has since been deleted, that DiGA can be tested by patients free of charge for 14 days. The start-up representatives also suggested extending the trial period of the fast-track procedure. This is because if a company can only present study results at the end of the trial phase, they will no longer be reviewed by the BfArM. The SVDGV welcomed the plans to explicitly include the entitlement to DiGA for pregnancy and maternity in the draft legislation. However, in order to improve their healthcare, the association believes that it should be made clearer that pregnant women and mothers are always entitled to a DiGA. In addition, the SVDGV would like to see normal pregnancies included via the corresponding ICD-10 code. The start-up representative also suggested that contraceptive apps should also be approved as DiGAs in future.
You can read more about this in the full statement.
4.2 National Association of Statutory Health Insurance Physicians
The National Association of Statutory Health Insurance Physicians (KBV) only briefly addressed DiGA in its statement on the DigiG. It advocated that the approval of DiGA in risk class IIb in the draft law should be accompanied by the exclusion of provisional approval without completed evidence. In addition, the KBV advocated the introduction of a general reservation of approval by the health insurance funds for the prescription of DiGA, as is also the case for other health services that do not require approval, such as home nursing care.
Read the full text of the statement on the draft law here.
4.3 National Association of Statutory Health Insurance Funds
The National Association of Statutory Health Insurance Funds also rejected the provisional inclusion of a risk class IIb app in the DiGA directory without proof of evidence, as provided for in Karl Lauterbach’s draft of the DigiG. However, the health insurance fund representatives already consider the so-called fast-track procedure for provisional approval prior to positive proof of coverage to be unsuitable as a whole.
The National Association of Statutory Health Insurance Funds supported the use of digital health applications to improve medical care for pregnant women. The extension of the entitlement to such applications concerns pregnant women with specific health problems, but not normal pregnancy support or preventive applications. Due to a lack of clarity in the existing legislation, the GKV-Spitzenverband called for a more precise definition of the purposes and conditions of use by the legislator. This should ensure that statutory health insurance benefits are specifically geared towards the needs of pregnant women with health problems.
More on this can be found in the statement of the National Association of Statutory Health Insurance Funds.
5. Conclusion
The DigiG sets the course for the future of digital healthcare. Overall, the DigiG is welcomed and seen as an important step towards digitalization in the healthcare sector. However, the law has mixed effects for DiGA manufacturers. The possibility of approving DiGAs in risk class IIb is a positive development. At the same time, the exclusion of these DiGAs from provisional approval and the mandatory performance measurement pose challenges for manufacturers. The continuing lack of clarity regarding DiGA in pregnancy could also prove to be an obstacle. The exact effects will only become apparent over time.




