Many manufacturers of medical software and health apps are faced with the question: How can digital solutions be used to build a sustainable business model?
The market for self-paying patients in the German healthcare system is relatively small, and the path to reimbursement by health insurance companies is challenging.
Although there are established reimbursement channels, such as inclusion in the DiGA directory or individual remuneration via selective contracts, these access routes can be lengthy, complex, and costly.
It is therefore worth considering alternative financing options while working toward inclusion in standard care. One such alternative for financing and reimbursing digital health solutions is the innovation fund under Section 92a of the German Social Code, Book V (SGB V).
In this expert article, we explain …
- what opportunities the Innovation Fund offers,
- which requirements must be met in order to receive funding,
- how high the chances of success are for software products,
- and for which types of digital concepts applications can be submitted.
Update – December 2025: In December 2025, BASYS took stock of ten years of “new forms of care” in the Innovation Fund. We summarize the most important findings in Chapter 6.
Table of Contents
- 1. Definition of the Innovation Fund
- 2. Difference between funding & reimbursement
- 3. Requirements for funding from the Innovation Fund
- 4. The application procedure for the Innovation Fund
- 4.1 What application procedures are there?
- 4.2 Which application procedure is right for my project?
- 4.3 What are the requirements for an application?
- 4.4 What are the criteria for funding?
- 4.5 Example of a project funded by the Innovation Fund: INTEGRATE-ATMP
- 4.6 What happens after the project ends?
- 5. Which software products are eligible for innovation fund funding?
- 6. Preliminary Assessment after 10 years of the Innovation Fund: What is actually reaching the Healthcare System?
- 7. Conclusion: Is the Innovation Fund worthwhile for Medical Software Manufacturers?
- 8.
1. Definition of the Innovation Fund
1.1 What is the G-BA Innovation Fund?
The Innovation Fund was established at the beginning of 2016 by the GKV Care Strengthening Act and is based at the Joint Federal Committee (G-BA).
Its aim is to promote new forms of healthcare provision and healthcare research that go beyond the standard care currently available. Specifically, innovative approaches in healthcare are to be scientifically tested in order to make care more efficient, more patient-centered, and of higher quality. The fund has since established itself as a key instrument for testing (digital) innovations in healthcare under real-life conditions.
1.2 How much funding is available?
Since its launch, around €200 million in funding has been made available each year. The fund was originally set up as a temporary measure (until 2019, later extended to 2024), but following a positive evaluation, it was made permanent and will continue from 2025 onwards.
The majority of the budget (€160 million) will go toward projects for new forms of care, while €40 million has been set aside for care research.
1.3 Who manages the Innovation Fund?
The Innovation Committee of the Joint Federal Committee (G-BA) is responsible for this. It sets out thematic priorities and criteria in regular funding announcements and decides on applications.
Projects typically run for up to three (sometimes four) years and are accompanied by scientific support.
Once a project has been completed, the results are evaluated and forwarded to the relevant bodies to determine whether the innovation should be incorporated into standard care.
The Innovation Committee is made up of representatives from the G-BA’s sponsoring organizations (health insurance funds, associations of statutory health insurance physicians, hospitals) as well as the Federal Ministry of Health and the Federal Ministry of Research. It also includes independent experts from the fields of science and healthcare.
Composition of the Innovation Committee
1.4 Who is involved?
Typically, various stakeholders in the healthcare sector form consortia for innovation fund projects. These include, for example:
- Health insurance companies
- Hospitals and university clinics
- Medical networks or medical care centers
- Research institutions
- Private companies (e.g., in the field of digital health)
Together, they develop a care concept that is then tested under everyday conditions for several years. Digital health solutions are often a central component of this—for example, for coordination, communication, or diagnostics.
1.5 Overview of funding categories
The Innovation Fund distinguishes between two areas of funding in accordance with Section 92a of the German Social Code, Book V (SGB V):
- Category 1: New forms of care
- Category 2: Health services research
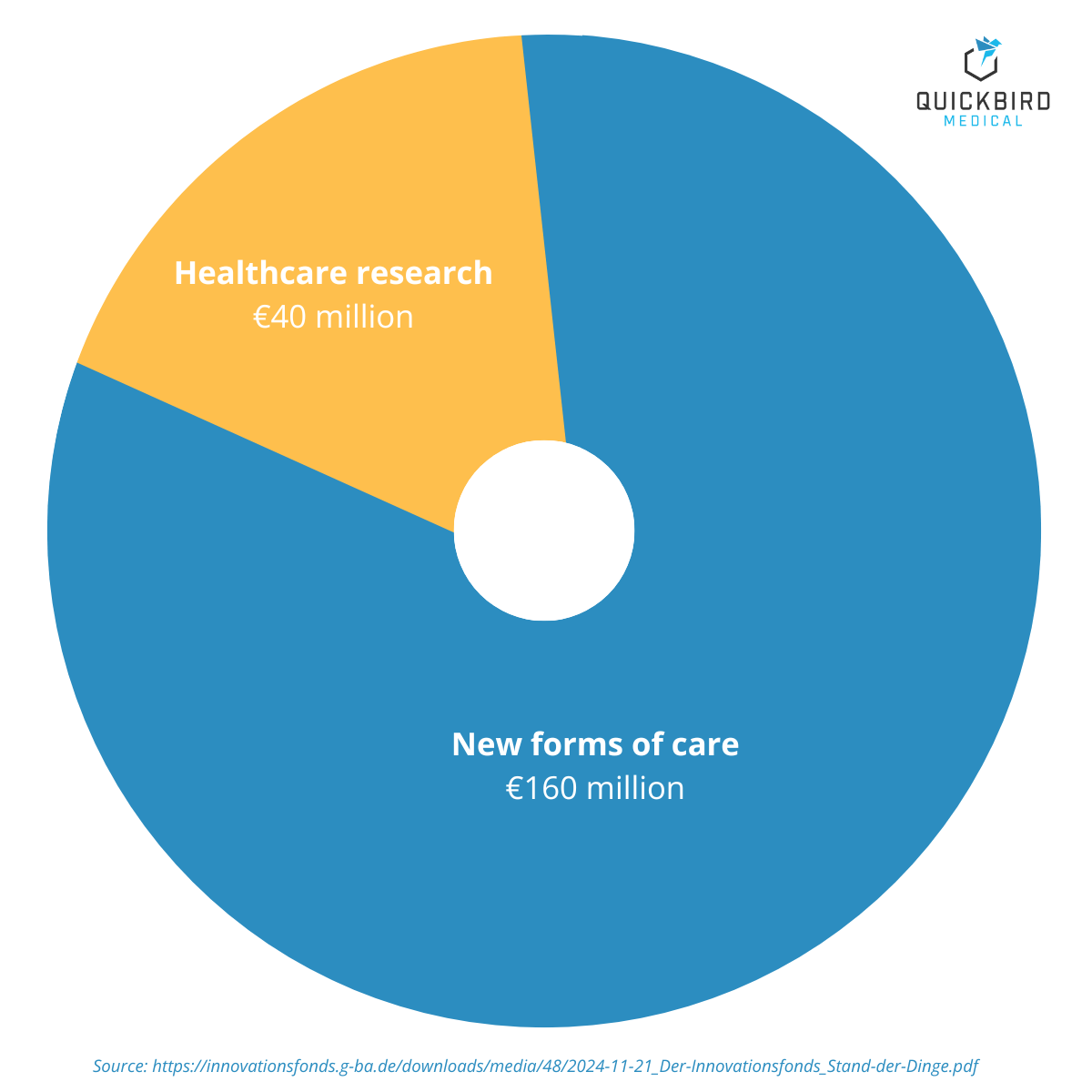
Funding categories and funding volume of the Innovation Fund (2025)
1.5.1 Category 1: New forms of care
This includes projects that test new care models or organizational processes in patient care. Digital health applications are often part of the concept, such as telemedicine platforms, digital communication tools, or AI-supported diagnosis and therapy management.
Important: These projects take place under everyday conditions in healthcare—for example, as pilot programs with real service providers and patients. The digital solution is always embedded in a healthcare concept, for example to optimize processes or overcome sector boundaries.
The whole process will be accompanied by a scientific evaluation to measure the effects on healthcare provision and patient care.
This category is particularly relevant for manufacturers of medical software, as it specifically evaluates completely new solutions and structures.
1.5.2 Category 2: Health services research
This area is not concerned with implementing new healthcare models, but rather with analyzing the current state of healthcare provision. Funding is available for scientific studies evaluating G-BA guidelines, guideline development, and health economic analyses, for example.
This category is only relevant for manufacturers if their own digital solution is already part of standard care and is to be evaluated as part of a study.
If, on the other hand, the product still needs to be brought to market, the project does not fall into this category but belongs to the ‘new forms of supply’ category.
Note: In this article, we focus exclusively on the funding area ‘New Forms of Care’, as this is significantly more relevant to medical software manufacturers in practice than the funding available in the area of healthcare research.
2. Difference between funding & reimbursement
When discussing the financing of digital health solutions, it is important to understand the difference between funding and reimbursement. Reimbursement – as is the case with DiGA, for example – means that the application is included in the SHI benefits catalog and every use is paid for by the health insurance company. This creates predictable revenues and is part of standard care.
The Innovation Fund works differently: it is not a permanent benefit, but rather temporary project funding.
For manufacturers of software products, funding from the Innovation Fund means that the costs of software implementation in the project are covered, whether as a contractor or consortium partner. And even if the software is not yet officially reimbursed by health insurance companies, money is already flowing through the G-BA funding pot.
At the same time, real healthcare data is generated, which can later serve as a basis for further reimbursement methods, such as selective contracts or remuneration via the DRG system.
3. Requirements for funding from the Innovation Fund
3.1 Which projects are eligible for funding?
Funding will be provided for new forms of care that
- aim in particular to further develop cross-sector care:
- Overcoming the separation of sectors
- Optimizing intersectoral interfaces
- Further developing selective contractual care
- demonstrate a viable evaluation concept, and
- have sufficient potential for long-term inclusion in the healthcare system (implementation potential).
Further information can be found here.
3.2 Which projects are not eligible for funding?
Not every project is eligible for funding from the Innovation Fund. There are a number of exclusion criteria that applicants should be aware of before investing time and resources. Here are the most important points:
- Economically driven product development: The Innovation Fund is not a funding program for traditional product development with a direct commercial objective. Projects in which commercial enterprises have a direct economic interest in the outcome are excluded, such as the development or testing of a product with the aim of subsequently selling it directly.
- Traditional R&D, regulatory or drug studies
- Research and development of medical devices or pharmaceuticals
- Clinical trials in accordance with EU Medical Device Regulation (EU) 2017/745
- Performance evaluation tests for in vitro diagnostic medical devices
- AMNOG studies on the early benefit assessment of medicinal products
- Studies to test new examination and treatment methods (Section 137e SGB V)
- DiGA and DiPA verification studies: Studies verifying positive effects of care within the framework of DiGA (Section 33a SGB V) or DiPA (Section 40a SGB XI) are also not eligible for funding. This verification must be provided separately via the BfArM or corresponding procedures and does not fall within the remit of the Innovation Fund.
- Projects already in progress or receiving other funding: Projects or plans that have already started and are already receiving other funding (e.g., from public funds) at the time of application are not eligible. The Innovation Fund does not fund duplicate structures or retroactive measures.
- Double applications are not permitted: You may only submit one application for each of the funding announcements for ‘New Forms of Care’ published in a given year. Double or parallel applications with the same project approach are not permitted.
4. The application procedure for the Innovation Fund
4.1 What application procedures are there?
The Innovation Fund distinguishes between four different funding formats in the area of new forms of care. These differ in terms of the topics covered, the process structure (one-stage vs. two-stage) and the project duration:
- Open-topic funding – Single stage, long
In this procedure, a complete application is submitted directly. The project must already be comprehensively planned, including an evaluation concept, a time and financial plan, and a consortium.
- Open-topic funding – Single stage, short
This format is aimed at smaller, faster-starting projects with a maximum duration of 24 months. Here, too, the application is submitted directly and completely in one step.
- Topic-specific funding – two-stage long
There are two phases in this process:
- Stage 1: Concept phase – submission of a rough idea on a given topic.
- Stage 2: Full application – A complete funding application is submitted with a fully developed care concept, specific care objectives and implementation plan, a validated evaluation concept, a time and financial plan, and binding commitments from all consortium partners.
This method is suitable for complex projects with extensive planning requirements.
- Open-topic funding – two-stage long
Here, too, the procedure is two-stage:
- Stage 1: Concept phase – rough sketch of ideas without any specific theme.
- Stage 2: Full application – as for the topic-specific variant.
This funding line thus offers scope for innovative ideas that require greater development effort.
Please note: All procedures require that the application documents are submitted in full and on time. The documents required (e.g., project description, consortium agreement, financing plan, ethics concept) are specified in detail in the respective funding announcements.
→ Link to the current tenders:
https://innovationsfonds.g-ba.de/foerderbekanntmachungen/
4.2 Which application procedure is right for my project?
When which application is most appropriate depends on the maturity of the project idea.
- Mature project status:
- The project idea has been fully developed.
- A well-developed supply concept is in place.
- Consortium partners (e.g., health insurance funds, healthcare providers) are bound by the agreement.
- The evaluation concept, schedule, and budget have been planned in detail.
➔ single-stage process
- Less mature project status:
- The basic idea is there, but it has not yet been worked out in detail.
- There is still a lack of validated partners, concrete evaluation, and precise implementation planning.
- The aim is to refine the project idea in a six-month concept phase (stage 1).
- After that, a full application can be submitted in stage 2.
➔ two-stage process
Comparison of the single-stage and two-stage procedures:
| Criterion | Single-stage (long/short) | Two-stage (long) |
| Project idea | Fully developed | still in the planning stage |
| Partners | specific commitments | still unclear / in preparation |
| Time required before start | Shorter, no intermediate steps | 6-month concept phase required |
| Funding for the concept phase | no | Yes, level 1 is subsidized |
| Bureaucratic effort | lower | higher (2 application rounds) |
| Flexibility during planning | lower | higher (concept can still be adjusted) |
4.3 What are the requirements for an application?
In order for a project to be eligible for funding, a number of basic conditions must be met. These apply regardless of the application procedure:
- Legally compliant implementation: The planned form of care must comply with existing legal requirements—in other words, it must not implement anything that conflicts with medical professional regulations or SGB V (German Social Security Code V).
- Data protection & ethics: The processing of health data must comply with the requirements of the GDPR. Ethical standards are also expected to be observed, for example in studies involving patients or in the processing of sensitive data.
- Scientific standards: The project must be scientifically sound in terms of both its care component and its evaluation. Standards from care research and empirical study design apply.
- Interoperability & interfaces: Digital solutions must be compatible with other systems in the healthcare sector. Connection to the telematics infrastructure (TI) of gematik is particularly relevant.
- Make results accessible: The evaluation results must be published transparently, regardless of whether the project was successful or not. This is not about disclosing internal plans or outlines, but rather about publishing the results in a comprehensible manner after the project has ended.
- Participation in meta-evaluations: The results of individual projects should be incorporated into an overall assessment of the funding strategy. To this end, the G-BA may request participation in cross-cutting evaluation formats, for example to analyze which types of innovations prove successful in the long term.
4.4 What are the criteria for funding?
Provided that all of the above-mentioned basic eligibility requirements are met, the specialist evaluation will be based on the following eligibility criteria:
- Relevance
- Improvement of supply
- Improvement in the quality and/or efficiency of care
- Remedying supply shortages
- Optimization of cooperation within and between different care areas, care facilities, and professional groups
- Interdisciplinary and cross-disciplinary care models
- Implementation potential
- Transferability of findings, particularly to other regions or indications
- Evaluability: methodological and scientific quality of the evaluation concept
- Feasibility of the project during its term
- Proportionality of implementation costs and benefits
- Patient involvement
The eligibility criteria are described in detail each year in the respective funding announcements.
The decision itself is made in two stages:
- Evaluation by a pool of experts: First, independent experts from the ‘expert pool’ evaluate the applications submitted. The expert pool is re-elected every two years and consists of ‘individuals who have practical or scientific experience in (digital) healthcare and who come from a variety of sectors and professions’.
- Decision by the G-BA: Based on the application and the recommendations of the expert panel, the G-BA’s Innovation Committee decides which projects will receive funding.
4.5 Example of a project funded by the Innovation Fund: INTEGRATE-ATMP
A concrete example of the successful promotion of a digital health solution is the INTEGRATE-ATMP project, which was initiated by Heidelberg University Hospital. QuickBird Medical was responsible for the technical implementation of the software platform in this project, working closely with all parties involved, and has successfully completed this task.
Thanks to €13.6 million in funding from the Joint Federal Committee’s (G-BA) Innovation Fund, INTEGRATE-ATMP is paving the way for harmonized structures in patient care. The telemedicine communication platform enables structured and direct communication between clinics, treating physicians, and patients. The aim is to relieve the burden on patients in their everyday therapy and on their treatment centers in their everyday care. A telecommunications platform has been developed for this purpose.
You can find more information here: https://quickbirdmedical.com/project/integrate-atmp/
Further examples of projects funded by the Innovation Fund:
- BlenCon – Blended Consultation
- Rise-uP – Innovative back pain therapy with e-health
- Telemedical emergency services in Bavaria
- TELnet@NRW – Telemedical, intersectoral network
- WELCOME – Digital transition to post-hospital care
4.6 What happens after the project ends?
At the end of each funded project, the G-BA is faced with a key question: Has the idea proven itself – and does it belong in standard care on a permanent basis?
The G-BA has three months after submission of the evaluation report to carry out this review. The decision is published as an official resolution on the website, including a proposal on how and by whom implementation can take place.
Typically, self-governing bodies are responsible for this, e.g., the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds) or the Kassenärztliche Bundesvereinigung (KBV, National Association of Statutory Health Insurance Physicians). Their feedback is also publicly available, which makes it possible to see whether a project has really had an impact.
Unfortunately, there is currently no central list where you can see at a glance which projects have been adopted into standard care or have received a recommendation for implementation. However, since 2024, the agencies mentioned are required to inform the G-BA about the outcome of the project proposals. This feedback can be found on the individual project pages—for example, for the Sisyphos project.
The transition to care can happen in various ways: through legal changes (e.g., in the SGBV), through contracts, through new guidelines, or through recommendations.
To date, only one project has been directly approved for inclusion in the coverage — but this decision had to be withdrawn later because the project team revised its findings. The press releases on this can be found here.
5. Which software products are eligible for innovation fund funding?
Not every digital health solution is equally suited to the Innovation Fund. Given the effort involved and the requirements, it is particularly worthwhile applying for certain types of products and stages of development. The following subsections provide examples of this.
5.1 Complex supply solutions – no individual software products
Products that deeply integrate into care processes are more important than individual, standalone software products. Examples include telemedicine network solutions, cross-sector platforms, and digital systems for coordinating between general practitioners and specialists or between clinics and outpatient care. Such solutions only reveal their full value when multiple players work together—making them ideal for a consortium. Individual apps, on the other hand (such as a pure self-management app without the involvement of doctors), have fewer points of contact with a care model and are therefore less likely to be the subject of innovation fund projects.
5.2 Products with high evidence requirements
If a piece of software is new and still viewed with scepticism by decision-makers, an innovation fund project can provide the necessary evidence. There are often high barriers to reimbursement, particularly for AI-based diagnostic tools, digital therapies for serious illnesses or software classified as a high-risk medical device (IIb/III). Here, scientific support within the framework of funding can provide evidence that the product is safe and effective. This makes it easier to obtain a positive G-BA decision or an entry in the list of medical aids, for example.
5.3 Niche indications and rare diseases
Digital applications for rare diseases or very small patient groups are difficult to finance through the DiGA fast track, but the Innovation Fund offers them an opportunity to be tested. Rare diseases often involve high development costs and a limited market. The Innovation Fund can step in here and finance pilot projects that test care solutions for orphan diseases. One example is the TRANSLATE-NAMSE consortium project, in which several university hospitals and health insurance funds are working together to improve care for people with rare diseases—funded for 42 months with around €13.4 million. In such cases, the Innovation Fund serves as a test laboratory for niche applications that would otherwise have no chance of receiving funding due to the low number of cases.
5.4 Supply management tools
Care management tools—such as follow-up apps, triage systems, and medication management platforms—often offer indirect benefits for the healthcare system, but are difficult to implement in routine practice. Since such solutions primarily prevent secondary diseases or make processes more efficient, they do not always fit into existing reimbursement structures. A prominent example is the AdAM medication management project for polypharmacy patients: initiated by a health insurance company and an association of statutory health insurance physicians, it received around €16 million in start-up funding from the Innovation Fund.
6. Preliminary Assessment after 10 years of the Innovation Fund: What is actually reaching the Healthcare System?
In December 2025, BASYS Consulting for Applied Systems Research published an evaluation of the first ten years of “New Forms of Care” in the Innovation Fund. The results provide insightful information about the results achieved so far and are also relevant for manufacturers of digital health solutions.
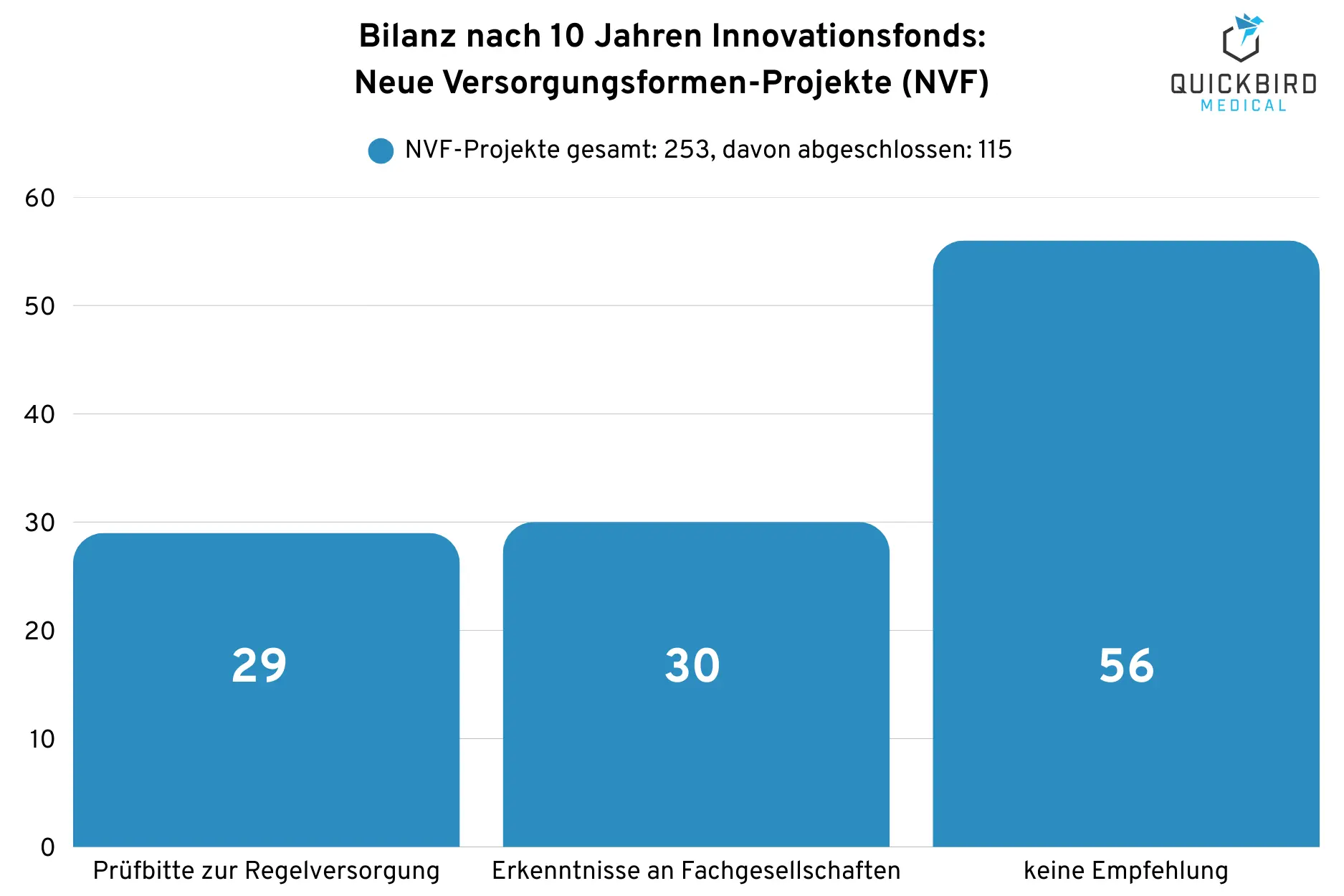
In the “New Forms of Care” (NVF) funding area, 115 of 253 projects were completed in the first ten years. Of these 115 projects:
- 29 projects received a request for review for transfer to standard care
- The findings from 30 projects were shared with professional associations.
- 56 projects did not receive a recommendation
Review of completed projects involving new forms of care (NVF) after 10 years of Innovation Fund fundinga
Important: “No recommendation” does not automatically mean “useless”; the results can still help to further develop care models and their cost-effectiveness.
The outcome is exciting: among the 29 projects recommended for implementation, there are five with proven, significant cost savings:
- CoCare with a statistically significant reduction of 1.6 hospital days (cross-sector coordination of services for people in need of care)
- IGiB-StimMT (Restructuring of healthcare provision in rural areas)
- Rise-uP (improved pain therapy)
- PROMoting Quality (quality assurance after hip replacement)
- STEP.De (sports therapy)
At the same time, it is clear why evidence is often difficult to obtain: in several projects, the number of cases was insufficient due to high dropout rates during the coronavirus pandemic, and there were often only “tentative” savings, and in some cases even higher costs in the intervention group. Nevertheless, according to BASYS, all 29 projects with implementation recommendations showed an improvement in outcomes, 5 of which showed significant cost savings and 4 of which showed potential cost savings. Among the projects with potential cost savings are, for example, these two with a digital focus:
- Eric (multidisciplinary, telemedicine consultation)
- pAVK-TeGeCoach (telemetrically supported walking training)
7. Conclusion: Is the Innovation Fund worthwhile for Medical Software Manufacturers?
The Innovation Fund offers software manufacturers an opportunity to obtain funding outside the traditional reimbursement channels and to test their product in a real-world healthcare setting. It can serve as a catalyst, particularly for innovative solutions seeking a place in the healthcare system. The funding generates evidence and experience that can later pave the way for entry into standard care, for example via DiGA, selective contracts, or other reimbursement channels.
The interim results after ten years underscore this: the 29 NVF projects recommended for implementation have demonstrated an improvement in the quality of care. And the BASYS evaluation makes it clear that the involvement of industry, especially in the area of digital solutions and AI, is expressly called for. Software manufacturers who want to help shape new care models will find the Innovation Fund to be a politically desirable instrument.
However, funding is not an easy or short-term process. Between 2016 and 2021, over 2,000 applications were submitted and just over 500 projects were approved. The probability of success was roughly 20 to 25 percent. Manufacturers should be aware that
If you are looking for an implementation partner for your software or app project, please contact us. We specialize in the development and approval of digital healthcare solutions. QuickBird Medical has developed software products such as INTEGRATE-ATMP for customers that have been successfully funded by the Innovation Fund.
8. Further Information on the Innovation Fund
The contents of this article are based on the current status of public funding information and practical experience with the Innovation Fund. As framework conditions and requirements are subject to regular change, we recommend that you also refer to the official documents of the Innovation Committee at the G-BA.
A good place to start is the guide to applying for new forms of care, which is regularly updated by the G-BA: Go to guide
Current presentations and information on the latest funding opportunities are also available on the official website: https://innovationsfonds.g-ba.de