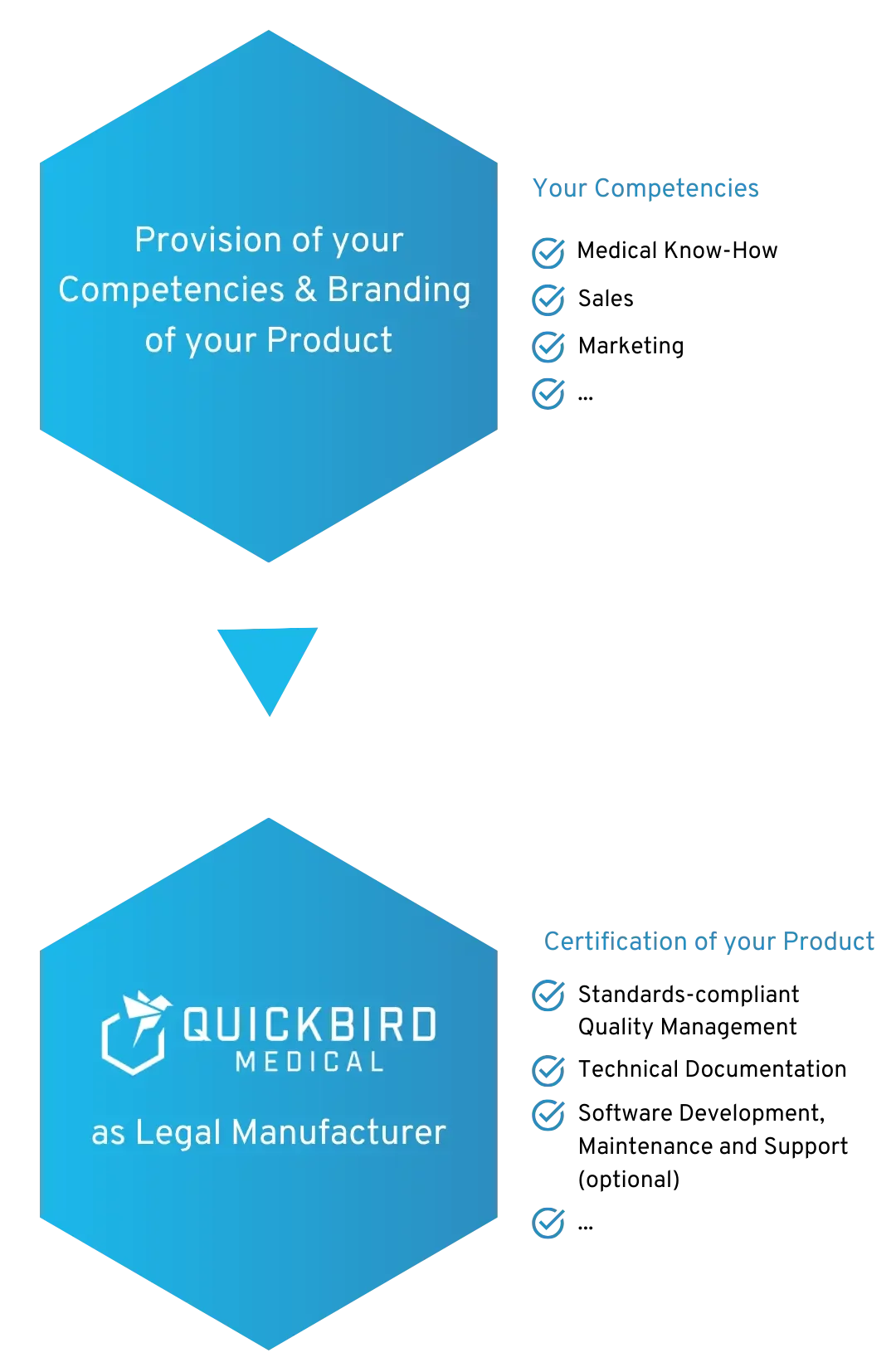
We will be the Legal Manufacturer for your Medical Device Software
Specialised Legal Manufacturer for Medical SoftwareWe assume manufacturer responsibility for your medical device software, medical app or DiGA. This includes ensuring overall regulatory conformity in accordance with MDR, DiGAV and ISO standards as the so-called legal manufacturer of the medical device.
We become the Legal Manufacturer for your Medical Device Software and/or DiGA
You are able to concentrate on your core competence and retain full control over your product. At the same time, we ensure compliance with the regulatory requirements for your MDR medical device. This means that you do not have to set up a quality management system (QMS) at your company and are not held back by regulations.

Certified according to ISO 13485 and ISO 27001

Specialized Expertise for Software Products

We are a team of 30 regulatory and medical device software experts. We have been developing software as a medical device and DiGA for more than 10 years. As a manufacturer of medical devices, we are therefore very familiar with the individual challenges of complex medical software, apps and digital health applications (DiGA).
How we launch your product onto the market efficiently and safely. In the process we accompany and advise you on all relevant topics.
If required, we can also take over the (further) development, operation and technical support for your software.
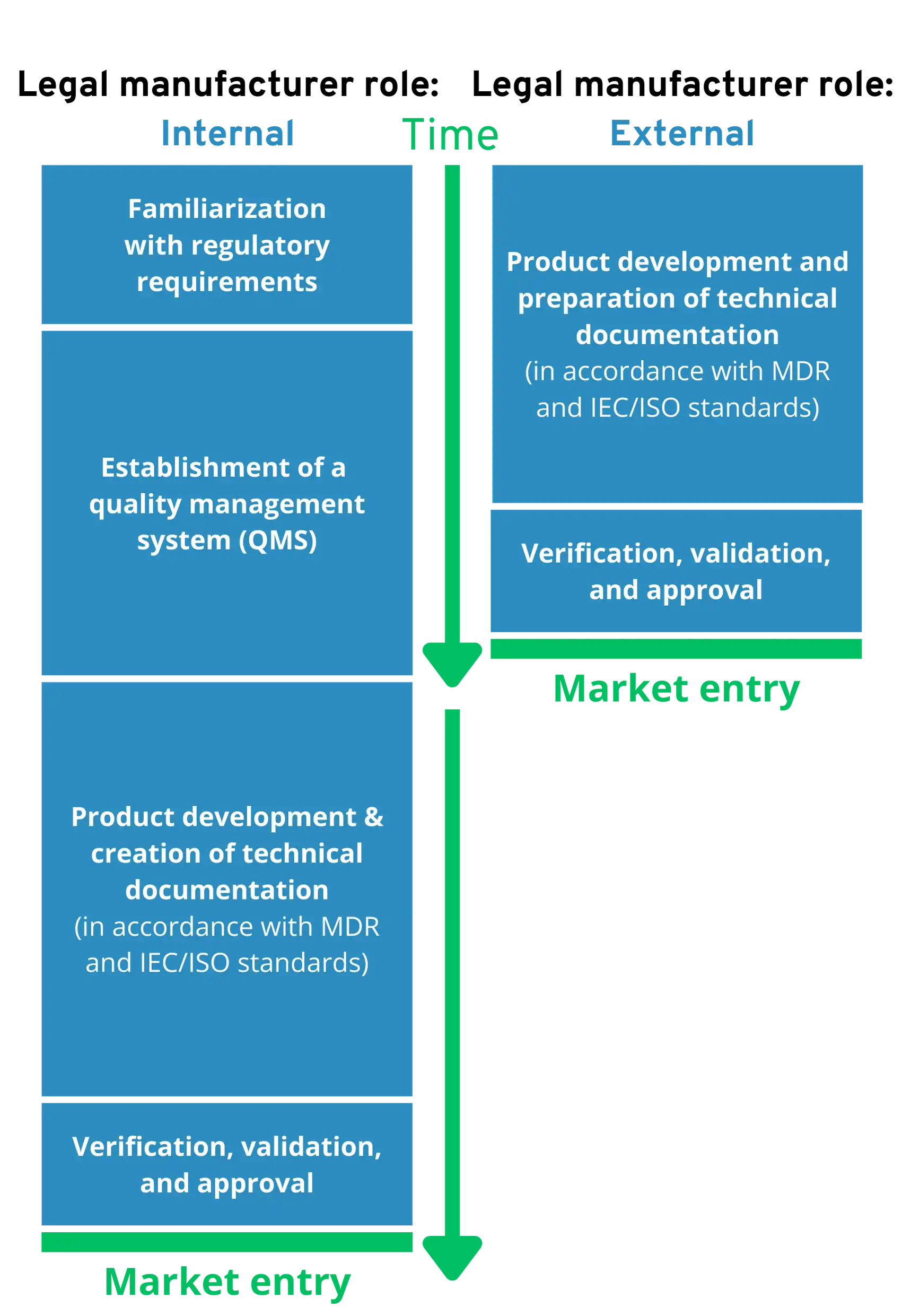
Comparison: External vs. Internal Legal Manufacturers
When is it worthwhile and when is it not worthwhile to outsource the role of the legal manufacturer?
External legal manufacturer if…
- You want to bring your product to market as quickly as possible in order to generate sales.
- You want to avoid regulatory complexity and internal efforts that distract from your core business.
- You want to outsource legal liability risks for the medical device.
Internal legal manufacturer, if…
- You already have a lot of regulatory expertise in-house and a quality management team that is familiar with software medical devices.
- You already have several software medical devices on the market.
In our technical article “Approving software medical devices without quality management and regulatory requirements?”, we answer the most important questions surrounding the topic of “external legal manufacturers” and examine which companies this model really makes sense for.
Your Benefits
Maximum safety for your medical device
Thanks to our many years of experience, we can bring your product to market safely. This eliminates unnecessary regulatory risks for your company.

In-depth consultation for your SaMD

Faster market access

Concentration on core competence
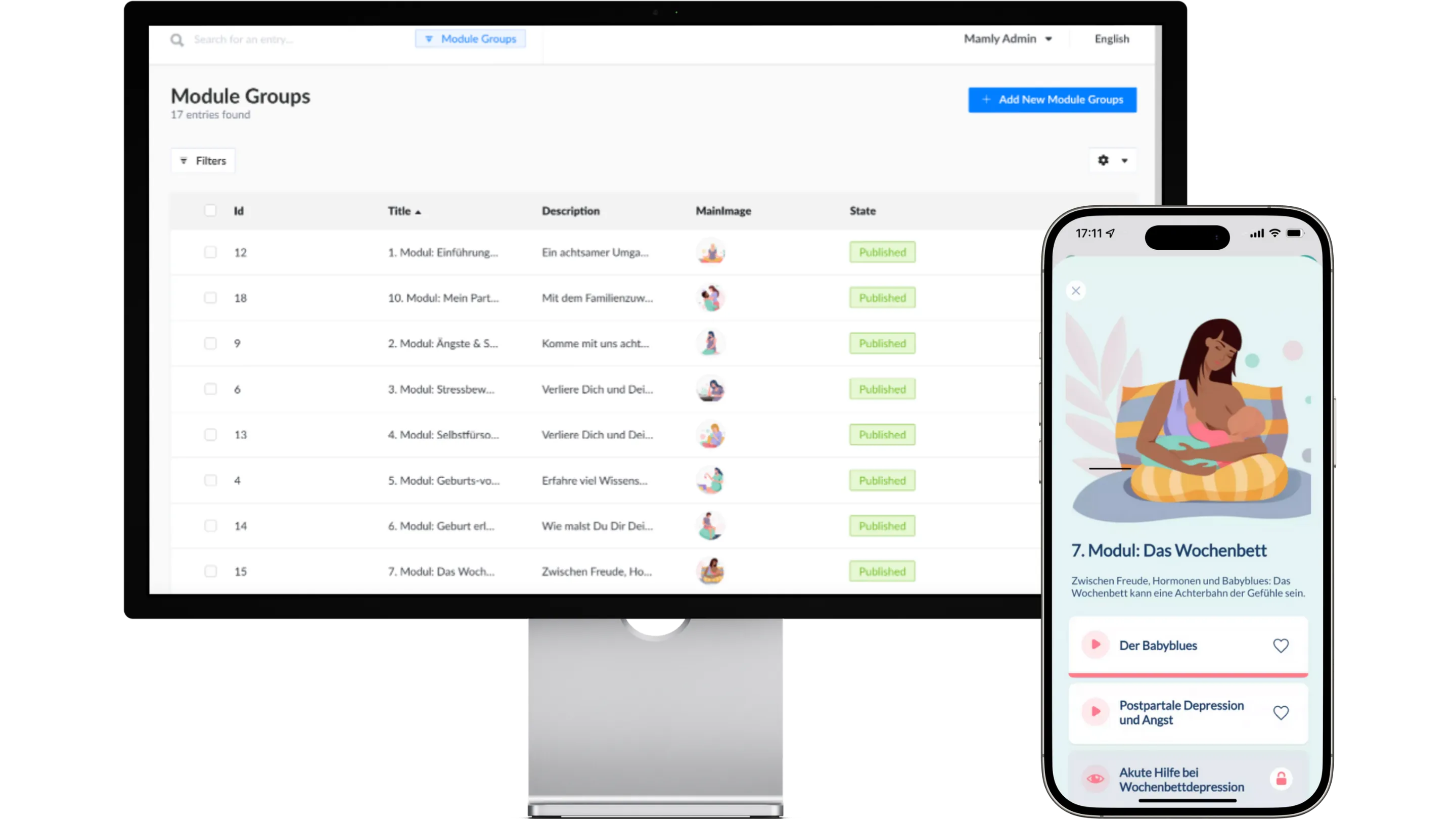
Mamly is a medical device app for the mental health of pregnant women. Find out here how we take on the role of the Legal Manufacturer for this successful product.

kontina
How we support Apogepha
as a Legal Manufacturer
kontina is a medical device for treating the symptoms of an overactive bladder (OAB). The app helps users to promote and improve their own bladder health in the long term. Read here how we are taking on the role of the Legal Manufacturer for this successful product.