Healthcare software manufacturers in Germany face a key challenge: How can digital solutions be used to generate revenue in the healthcare system? The market for privately paid products is small, and patients are often reluctant to pay for software out of their own pockets. At the same time, the familiar routes to reimbursement, such as inclusion in the DiGA directory, selective contracts, or DiPA classification, are lengthy, complex, and often only suitable for certain product categories.
The reality for many digital health companies is that without reimbursement from statutory health insurance providers, it is difficult enough to earn enough money in the German healthcare system to survive as a company in the long term. The question many manufacturers are therefore asking themselves is: Are there other ways to open the door to reimbursement besides the familiar models?
One approach that has received little attention to date is the listing of software in the GKV’s directory of medical aids (HMV). Originally intended for physical products such as walkers, hearing aids, and prosthetics, this raises the intriguing question of whether digital solutions can also follow this path—and whether it is worth the effort to position a software product as a ‘medical aid’.
In this article, we show why the list of medical aids is a potential route to reimbursement for software products, what requirements digital applications must meet, how the inclusion process works, and whether it is worthwhile for manufacturers.
Table of contents
- 1. Advantages for manufacturers listed in the medical aids directory
- 2. Definition of a medical aid
- 3. The GKV medical aids directory
- 4. Steps for approval in the medical aids directory
- 5. Qualification: Suitability of your software for the list of medical aids
- 6. Requirements for software in the list of medical aids
- 7. Application for inclusion in the list of medical aids
- 8. Remuneration and pricing with health insurance companies
- 9. Updating of product groups
- 10. Examples: Listed software products in the list of medical aids
- 11. Why is there hardly any standalone software in the list of medical aids? (Obstacles)
- 12. Further avenues for reimbursement by health insurance companies
- 13. Conclusion: Is the list of medical aids worthwhile for software manufacturers?
1. Advantages for manufacturers listed in the medical aids directory
The listing of a product in the GKV list of medical aids offers you, as a manufacturer, a key advantage over selective contracts, for example: All statutory health insurance companies in Germany are obliged to reimburse your product. This means that you do not have to convince each health insurance company separately about your product, but can follow a centralized application process.
However, before the actual reimbursement of costs takes place, manufacturers must clarify the amount of remuneration. There are two options here: Either the manufacturer joins an existing contract for a product group—this guarantees rapid market access but may mean fixed reimbursement rates that are not always ideal for software products. Alternatively, individual agreements can be made with each health insurance company, but this is time-consuming and resource-intensive.
This is a crucial difference to the DiGA procedure, in which the reimbursement amounts are negotiated once with the GKV-Spitzenverband (GKV-SV) and then apply to all health insurance funds.
| Aspect | List of medical aids | DiGA | Selective contracts |
| Guaranteed access to all health insurance companies | Yes, application permitted Reimbursement by all health insurance companies | Yes, application permitted Reimbursement by all health insurance companies | No, selective contract must be negotiated individually with each health insurance company |
| Centralized remuneration negotiations | No, remuneration to be agreed individually with each health insurance fund or existing contracts to be joined | Yes, remuneration level negotiated centrally with GKV-SV and standardized | No, remuneration to be agreed individually with each health insurance fund |
Nevertheless, listing in the list of medical aids remains an attractive option for making digital products available to insured persons across the board. However, this requires that your product falls within the definition of a medical aid.
2. Definition of a medical aid
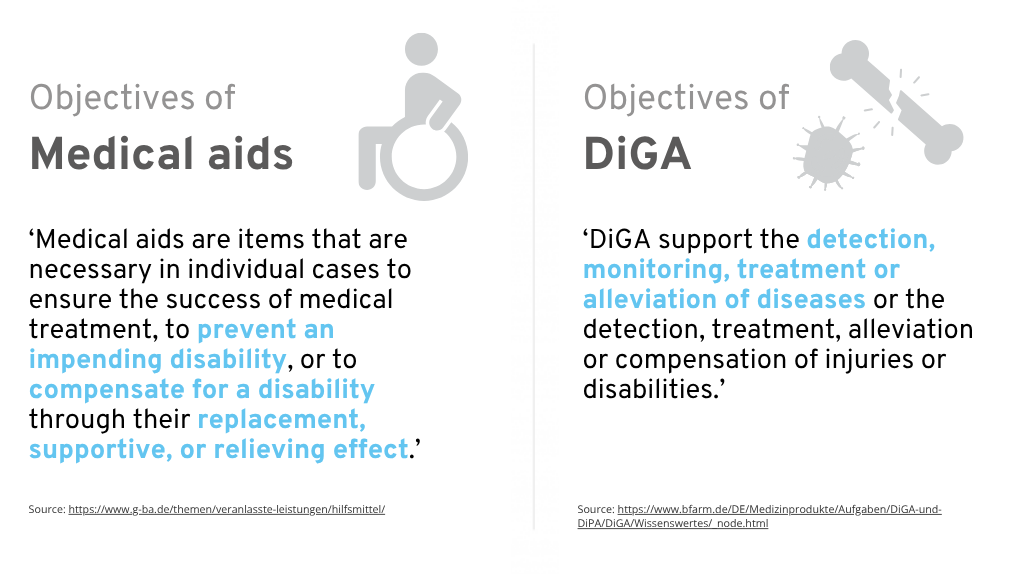
According to the definition of the Joint Federal Committee (G-BA), medical aids are items that ‘are necessary in individual cases to ensure the success of medical treatment, prevent an impending disability or compensate for a disability through a replacement, supportive or relieving effect’.
Medical aids can serve as ‘body replacements’ (§33 SGB V), among other things. Their primary purpose is therefore to help people perform a specific activity, compensate for a limitation or achieve a goal that would be more difficult or impossible without the aid.
General everyday items – such as a thermometer – cannot be considered aids. Nor can aids excluded under Section 34 of SGB V.
Classic aids include wheelchairs, hearing aids, incontinence aids, compression stockings, and prostheses. These are often physical objects. However, pure software products or apps can also fall under the definition of an aid. This was confirmed by the Federal Social Court for the first time in 2001.
Difference between the objectives of DiGA and medical aids
To sum up, the goal of medical aids is
… to ensure the success of medical treatment (Section 33 SGB V),…
… to prevent an impending disability (Section 33 SGB V and Section 47 SGB V)
… to compensate for an existing disability (Section 33 SGB V and Section 47 SGB V)
… to prevent the need for long-term care (Section 47 SGB V).
Reimbursable medical aids are listed in the list of medical aids published by the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds).
3. The GKV medical aids directory
The list of medical aids is compiled by the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds) in accordance with Section 139 of the German Social Code, Book V (SGB V), and is updated regularly. It lists medical aids whose costs can be covered by statutory health insurance (GKV).
The list of medical aids is similar to a catalog. Among other things, it serves as a guide for insured persons and service providers by providing information on reimbursable medical aids and their type and quality.
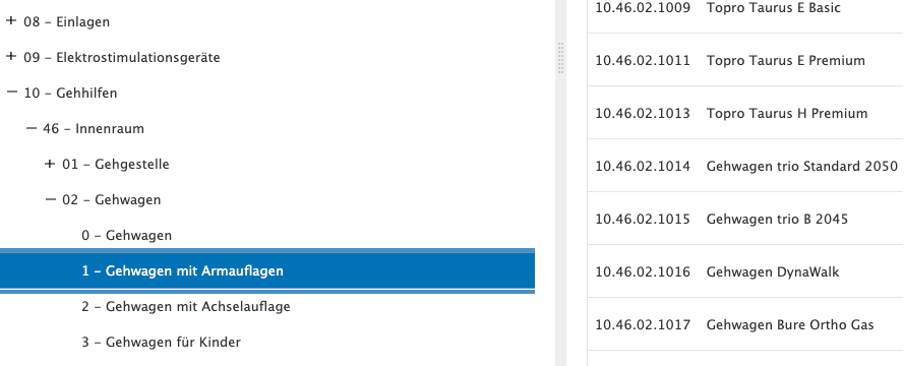
The GKV list of medical aids is structured as follows:
Product groups > Subgroups > Place of use > Product types > Products
Screenshot from the GKV list of medical aids
The list of aids (HMV) is publicly available at: https://hilfsmittel.gkv-spitzenverband.de/home
The numbering of products in the list of medical aids follows a systematic and hierarchical structure, which ensures clear classification and clarity. The number consists of several digits: The first two digits indicate the main product group, the next two digits specify the place of application and the subgroups. At the end is the four-digit, individual product number, with the first digit again classifying the product type.
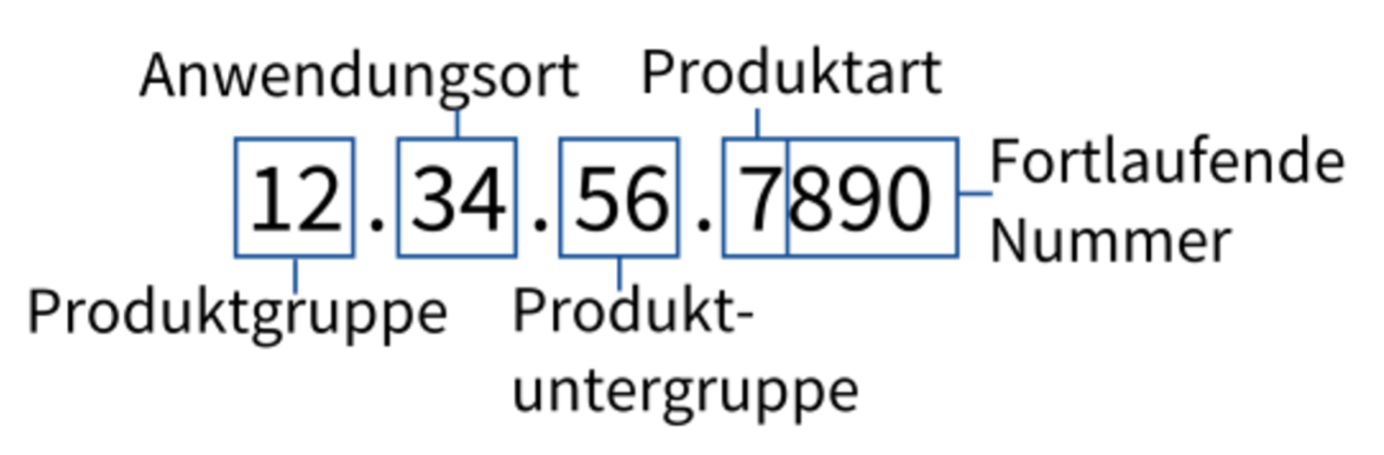
Example of product numbering in the list of medical aids –
Image source: https://www.rehadat-gkv.de/hinweise/aufbau-des-verzeichnisses/
4. Steps for approval in the medical aids directory
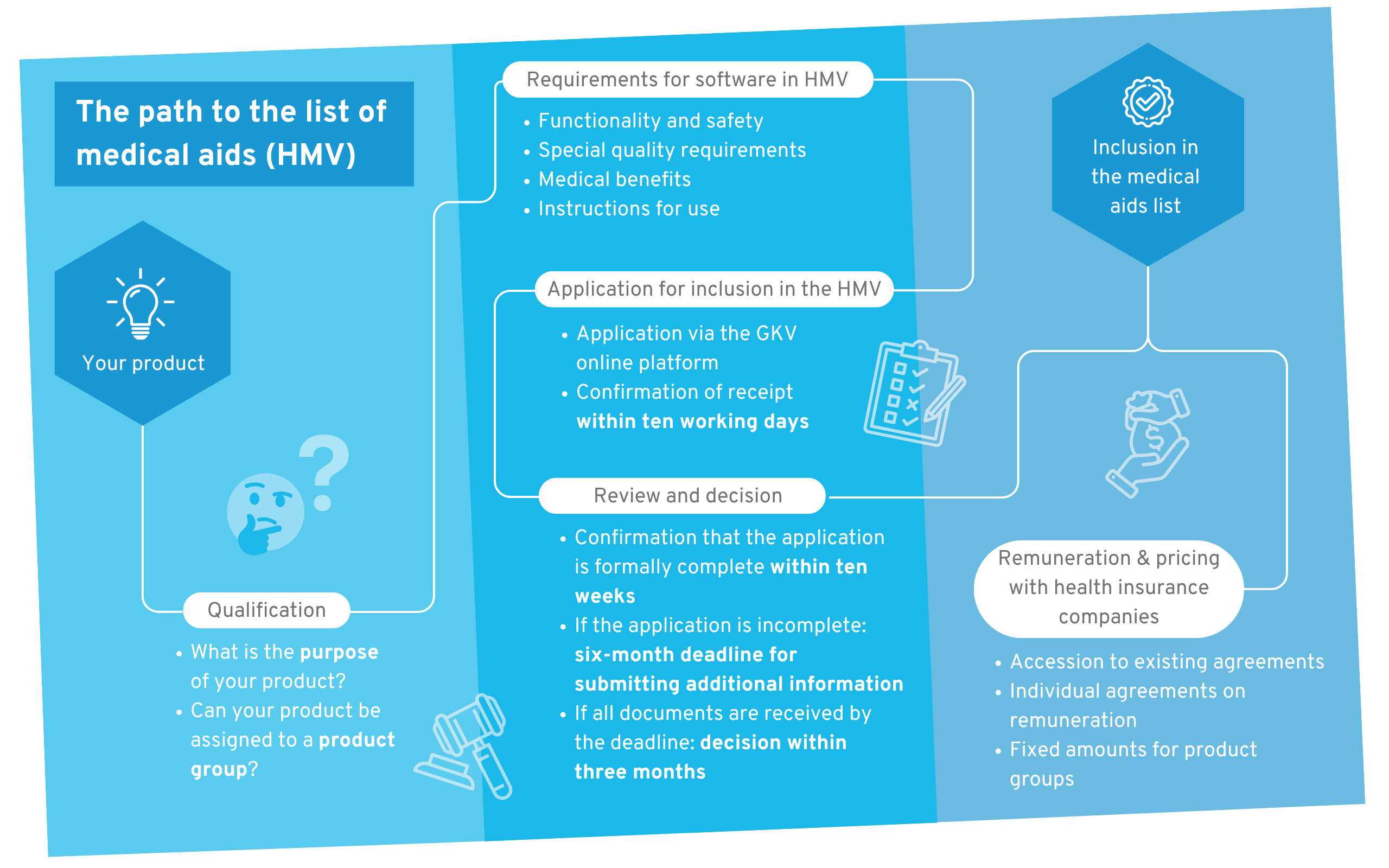
Process for applying for a software product for the GKV list of medical aids
The figure above outlines the process from start to successful compensation. The most important steps are as follows:
- Qualification: Is my product concept even suitable for inclusion in the list of medical aids?
- Compliance with requirements: You must implement all general and product-specific requirements necessary for inclusion in the list.
- Application: You must now complete the application and submit it.
- Review and decision: You will receive feedback and a decision regarding your application within the statutory time limits.
- Remuneration with health insurance: After successful admission to the list of medical aids, you must determine the amount of remuneration with the various health insurance companies.
After step 5, you are eligible for reimbursement and can generate revenue from your product. All steps of this process are explained in detail in the following chapters.
5. Qualification: Suitability of your software for the list of medical aids
To find out whether your product can be included in the list of medical aids, you should check the following.
5.1 Qualification check: Purpose of your product
We learned at the beginning that software products can also fall under the definition of ‘medical aids’ and can therefore be listed in the list of medical aids.
The big challenge for software products is to prove that the software fulfills its role as an assistive device—in other words, that it prevents or compensates for certain disabilities. It is important to understand that assistive devices are not therapies. They can and should, of course, accompany therapy in their function of ‘ensuring the success of medical treatment,’ but their primary task is to provide immediate assistance.
To find out whether your product can be listed in the list of medical aids, you must first check whether it meets the criteria for a medical aid. We have explained the definition of a medical aid in more detail in the section above.
5.2 Qualification check: Categorization into a product group
If your product potentially falls under the definition of a medical aid, we move on to step 2: You now need to find an existing product group in the list of medical aids that your product fits into.
First of all: Currently, there is no separate product group for standalone software products or mobile apps in the assistive technology directory.
This means: If you want to apply for software as an aid, you must look at the individual product groups and check the individual requirement profiles.
What if you don’t fit into any product group or type? If you do not fit into any existing product group, the only option left is to apply for a new product group. However, this process is extremely time-consuming and can potentially take many years. Therefore, this route is hardly desirable for software manufacturers.
The GKV stipulates the following regarding the inclusion of innovations in the list of medical aids:
‘New products are also being registered for inclusion in the list of medical aids that cannot be classified in the existing system because a corresponding product subgroup or type has not yet been created. Therefore, before such a product can be listed, the relevant product group must first be updated. This takes time, as all participation rights and procedural steps must be observed.’ (Translated from German)
You should therefore make sure to find an existing product group. If this is not possible, it may be advisable to choose a completely different reimbursement method, e.g. via selective contracts.
6. Requirements for software in the list of medical aids
Let’s first take a look at the legally defined requirements. The Social Security Code V describes in §139 paragraph 2 that a medical aid must be included in the list of medical aids
‘if the manufacturer has proven its functionality and safety, compliance with the quality requirements according to paragraph 2 and, if necessary, its medical benefit, and if it is provided with the information in German necessary for proper and safe handling’. (Translated from German)
On this basis, the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds) has defined specific, cross-product group requirements in its rules of procedure for compiling and updating the list of medical aids:
6.1 Requirement: Functionality and safety
For approved medical devices, functionality and safety are deemed to have been proven by the CE marking, although additional tests may be carried out in suspicious cases.
The definition of medical devices for the list of medical aids (HMV) refers to the Medical Devices Act (MPG) and not to the Medical Device Regulation (MDR). The reason for this is that the MDR also includes products that could be classified as medicinal products, which is too broad a framework for medical aids. However, the provisions of the MDR, including the conformity assessment procedures described therein, apply to the verification of functionality and safety in applications for inclusion.
For aids that are not medical devices, the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds) draws up specific requirements for functionality and safety in the individual product subgroups of the HMV. These can be found in the documents on the updates to the product groups.
A medical device does not necessarily have to be a certified MDR medical device.
6.2 Requirement: Special quality requirements
The specific quality requirements are determined based on the indication or application. The GKV specifies the relevant quality requirements and details on how to prove compliance with these requirements in the product subgroups of the HMV (including a description of the test procedures, if applicable) and the corresponding application forms. The exact requirements can also be found for each product (sub)group on the GKV website.
6.3 Requirement: Medical benefit
Proof of the medical benefit of an aid is required in accordance with Section 139 of the German Social Security Code V (SGB V) if the aid is not only intended to compensate for a disability but is also to be used as part of medically supervised treatment.
If the medical aid is inextricably linked to a new treatment method that is not yet recognized in outpatient care, it may only be included in the HMV once the Joint Federal Committee (G-BA) has given a positive assessment of the method.
If the GKV-Spitzenverband concludes that the medical aid is to be regarded as an integral part of a new method, it shall inform the manufacturer accordingly and at the same time request information from the G-BA.
If the medical device uses proven methods, scientific studies must confirm its medical benefits in accordance with the ‘current, generally accepted state of medical knowledge’. A single study is not sufficient. The method must be established.
6.4 Requirement: Instructions for use
HMV products must contain information in German to ensure safe and correct use. This information—specified in the HMV depending on the product group—includes instructions for use, indications, risks, contraindications, and instructions for operation, cleaning, disinfection, assembly, and materials, provided that the manufacturer has demonstrated compliance with these requirements.
7. Application for inclusion in the list of medical aids
Let’s now take a look at how long the process of inclusion in the list of medical aids takes and what the individual steps involve:
- Application: The application for inclusion must be submitted by the manufacturer or an authorized third party. This application can be submitted online via the GKV-Spitzenverband platform. Before doing so, it must of course be ensured that the necessary requirements (see above section) are met.
- Review: The GKV-Spitzenverband will send the applicant confirmation of receipt within ten working days of receiving the application and will determine within ten weeks whether the application is formally complete. This is solely a formal determination of completeness and does not reflect the completeness of the application’s content. If incomplete documents are submitted, there is a period of up to six months to submit the missing documents.
- Decision: If everything is submitted on time, the GKV will decide on the application within three months and inform the applicant of the outcome of the procedure.
- Success of inclusion: If the product is included, it is assigned an individual number and published in the list of aids and in the Federal Gazette.
Due to the complex evaluation process and any necessary queries, the entire process takes between six and twelve months on average.
8. Remuneration and pricing with health insurance companies
The inclusion of a product in the list of medical aids (HMV) is an important step, but does not guarantee automatic reimbursement. Before insured persons can obtain the product through their health insurance companies, the reimbursement amount must be clarified. Manufacturers have various options for achieving this.
8.1 Accession to existing agreements
Framework agreements already exist between health insurance companies or their associations and manufacturers of medical aids for many product groups. As a manufacturer of a medical aid, you have the option of joining these agreements if a corresponding agreement is available for your product group.
On the one hand, this option allows you to enter the market quickly, as no new negotiations with individual health insurance companies are necessary. The framework agreements regulate the details of care, including remuneration. On the other hand, the remuneration rates specified in the agreements may be inappropriate or too low for your product.
A point of contact for this is the Association of Substitute Health Insurance Funds (Verband der Ersatzkassen e. V., vdek). Manufacturers can join the contracts already negotiated by the vdek. More information about vdek contracts can be found here.
8.2 Individual agreements on remuneration
If no suitable contracts exist or you require special conditions, individual agreements can be made with the health insurance companies. This option offers more flexibility, but requires a considerable amount of time. Each health insurance company must be contacted individually and convinced.
Individual agreements are particularly useful if your product is unique and does not fit into an existing product group with framework agreements. According to Section 127 (3) of the German Social Code, Book V (SGB V), health insurance funds are obliged to conclude individual agreements if no suitable contracts exist and it is not possible to provide care for insured persons in any other way.
8.3 Fixed amounts for product groups
For many medical aids, the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds) sets fixed amounts that define the maximum reimbursement amount. Despite these fixed amounts, manufacturers must conclude contracts with health insurance companies or their associations or join existing contracts in order to be able to supply their products to insured persons. The fixed amounts serve as the maximum limit for reimbursement. However, it is also possible to agree on lower reimbursements in order to gain an advantage in price competition, for example.
Fixed amounts set the upper limit for statutory health insurance benefits and also the maximum entitlement of insured persons to care. If insured persons wish to receive care that exceeds the fixed amount, they must pay the difference out of their own pocket. The defined fixed amounts can be found on the GKV website.
9. Updating of product groups
Every five years, the basic continuation of the product group (and the requirements profile) is reviewed.
For manufacturers, this means: The GKV-Spitzenverband may request that you resubmit the “documents required for reviewing the HMV requirements” for the purpose of updating the HMV.
The GKV-Spitzenverband will inform you in writing of the supporting documents that are required and the deadline for submitting them.
If this check is not successful, your product will be removed from the medical aids directory along with its eligibility for reimbursement.
10. Examples: Listed software products in the list of medical aids
It is not easy to find standalone software aids—i.e., software that does not need to be connected to a specific physical device—in the list of medical aids.
However, you will find what you are looking for in product group 16, ‘Communication aids’. Subgroup 5, ‘Disability-friendly software for communication systems’, is particularly interesting here. There are currently (as of 2 December 2024) eight software products listed here:
- OnScreenKeys
- My-own-voice, version 2+
- OnScreenCommunicator
- WorldWide
- MULTiTEXT without voice output
- MULTiTEXT with voice output
- MULTiTEXT with on-screen keyboard without voice output
- MULTiTEXT with on-screen keyboard with voice output
Product group 99 – Miscellaneous:
- VisioCoach – Eye movement training
Even though this list is not exhaustive, unfortunately there are not many more standalone software products currently available in the list of medical aids. It should be noted that the list of medical aids has been in existence for much longer than, for example, the DiGA procedure at the BfArM. Nevertheless, there are hardly any standalone software or mobile apps in the list.
However, hardware-software combinations are very common. Product combinations, where, for example, a mobile app controls an electronic device, are found in large numbers in the assistive technology directory.
But why is there so little standalone software available?
11. Why is there hardly any standalone software in the list of medical aids? (Obstacles)
So why is it that relatively few standalone software products have made it into the list of assistive technology devices?
Reason 1: Lack of suitable product groups
Often, a product subgroup requires complete systems by definition—for example, an iPad including communication software or an emergency call system with a wrist tracker. This leaves little scope for incorporating a pure software solution into a product group.
The standalone software manufacturer would therefore have to apply for a new product subcategory. It can take many years to successfully create this product subcategory, which makes this process impractical for start-ups, for example.
Reason 2: Fixed maximum prices
The fixed amounts originally set for physical products or traditional medical devices often do not reflect the specific characteristics and value of today’s software products. For example, the high development costs, ongoing maintenance, and regular updates that are essential for software or mobile apps are not covered or taken into account here. For this reason, it may be unattractive or even economically unviable for manufacturers of software products to have their products listed in such categories. The fixed amounts set an upper limit for reimbursement, which may be significantly lower than the actual costs or market value of the software.
Reason 3: Complex process for individual agreements on the amount of reimbursement
Without joining an existing agreement, manufacturers must invest considerable additional resources in negotiating individual agreements, which increases financial costs and can delay market access.
Practical experience also shows: The chances of reaching an individual agreement with a health insurance fund, provided that suitable existing contracts exist for the product group, are extremely low. Furthermore, the resources required to negotiate an individual agreement should not be underestimated.
We would like to take this opportunity to thank Christoph Müller, Chairman of the Board of the German Federal Association for Electronic Assistive Devices (BEH), for providing us with a wealth of information and for his contribution to this article.
12. Further avenues for reimbursement by health insurance companies
In addition to the list of medical aids, there are several other ways in which software manufacturers can obtain reimbursement under the German healthcare system. These include:
- Overview of all paths to reimbursement: Read our white paper
- Digital health applications – DiGA procedure: To our guide
- Selective contracts with health insurance companies: Our guide
- Central Prevention Review Board (ZPP): About our guidelines
- Digital care applications – DiPA procedure: To our guide
In addition to the aforementioned reimbursement options, it is also worth looking into public funding opportunities that can bridge the gap. First and foremost, it is worth taking a look at the G-BA’s Innovation Fund, which provides financial support for digital healthcare solutions in their early stages.
You can find more information in our guide to the Innovation Fund: Go to guide
13. Conclusion: Is the list of medical aids worthwhile for software manufacturers?
In summary, it is worthwhile for manufacturers of medical software to take a look at the GKV list of medical aids.
It is important that your product is classified as an aid and fits into an existing product group. You should also check in advance whether fixed amounts apply to your product and whether these are sufficient for your company.
Under the right circumstances, this opens up a suitable route for reimbursement under standard care. For suitable products, this route is even significantly faster than inclusion in the now heavily regulated DiGA directory.
However, DiGA manufacturers could face challenges when seeking listing in the HMV. DiGA generally focuses on therapy and treatment-oriented goals, while the list of medical aids is primarily geared toward supportive applications that compensate for disabilities or limitations. This difference in focus often makes inclusion in the HMV less obvious for DiGA.
Products in which software controls an electronic, physical aid are also well suited for reimbursement as part of the aid directory.
If you are planning medical software and looking for a development partner, please contact us. As a specialized service provider, we focus on the regulatory-compliant development of medical apps and health software. We help you plan and implement a product that is ultimately reimbursable and successful in the healthcare market.