DiGA Development 2026: Your way into the BfArM directory
Are you planning to develop a DiGA? Then arrange a non-binding consultation now. We take care of software development, compliance with all regulatory requirements and support you on your way into the DiGA directory of the BfArM.
ORIKO®
DiGA that measurably help patients
We develop digital health applications (DiGA) for pharmaceutical companies such as Takeda and start-ups such as MiNDNET Solutions. The ADHD therapy app ORIKO® is the first DiGA for adults with ADHD to be listed in the BfArM directory.
Whitepaper for prospective manufacturers
In our white paper you will find the most important tips for DiGA manufacturers in 2026
- Development, approval, study and distribution of your DiGA
- Our experiences from numerous DiGA projects
- Practical advice for aspiring DiGA manufacturers
- The decisive risks for approval at the BfArM
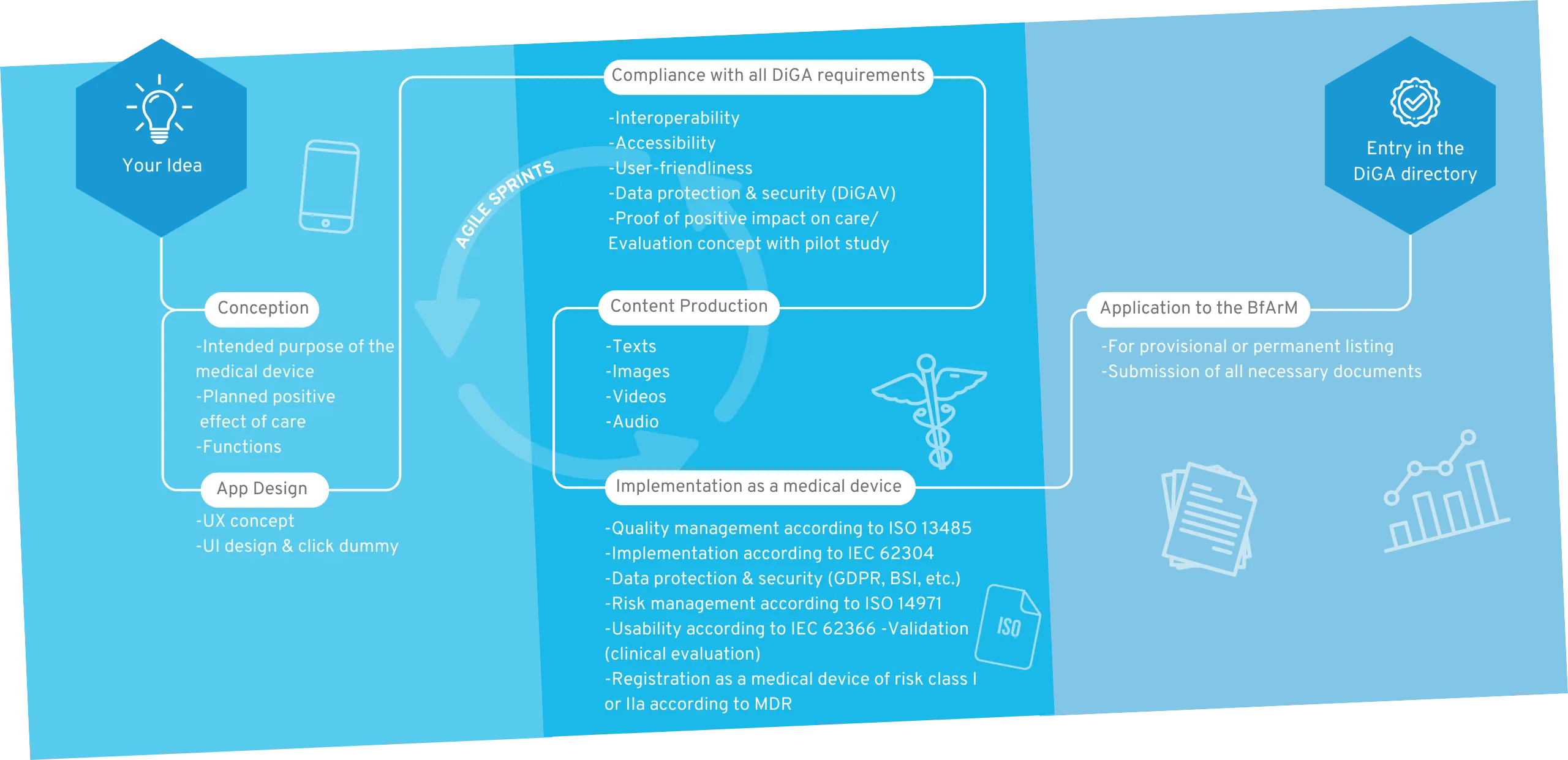
Your way into the DiGA directory
1. Conception
After the sparking idea, the conception begins, in which the target group, the benefit, the medical purpose and the requirements for your DiGA are defined.
UX and user research specialists
2. User Interface Design
A professional app design makes your app tangible for the first time and serves as an indispensable foundation for the implementation of the project. For visualization, we create wireframes and an initial click dummy, which is then filled with functionality.
3. MDR-compliant DiGA development
The regulatory jungle is obscure for many DiGA manufacturers at the beginning, because both the DiGAV and the MDR impose many requirements. In this step the functionalities of your DiGA are developed by us step by step, passing through our quality management processes according to ISO 13485 and IEC 62304.

4. Content Production
A medical app requires good content. Once the scientific evidence for the content is given, it is translated into multimedia formats. We support you with the creation of images, videos, texts and other visual elements for a unique user experience.
5. Implementation as a medical device
Your app will then be registered as a Class I, IIa or IIb medical device according to MDR. We will guide you through this process and assist you in finding a designated body if necessary.

6. BfArM approval
We support you with the application to the BfArM and prepare all documents. After the app has been tested by the BfArM with regard to safety, functionality, quality, data security and data protection (fast-track procedure), it is provisionally reimbursed by the statutory health insurance for a period of one year.
7. Inclusion in the DiGA directory
Now your DiGA will be listed in the BfArM directory and can be reimbursed by health insurers. We take care of regular software updates and the maintenance of your product.
Additionally, we offer advice on marketing your DiGA to reach more patients.
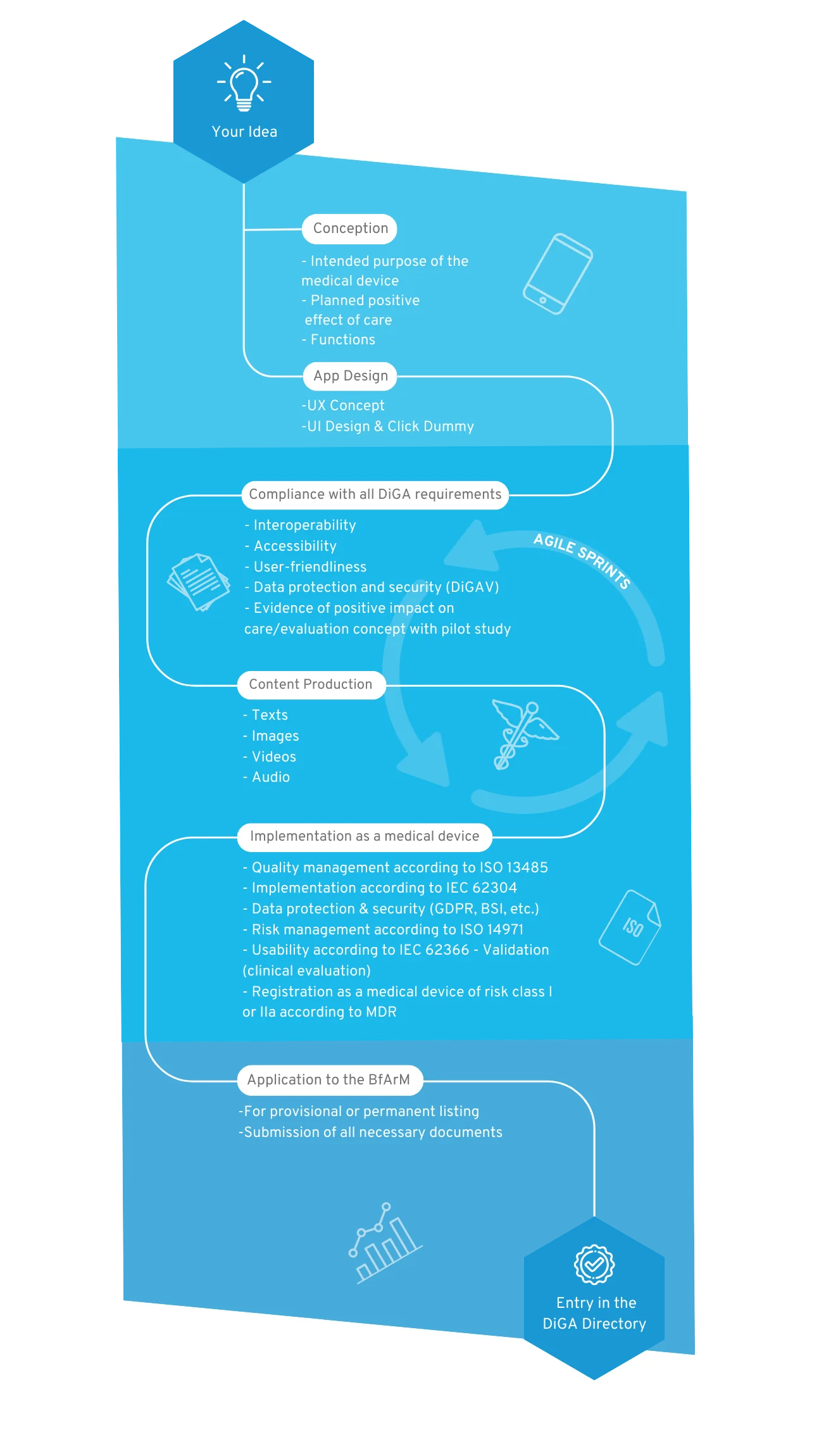
Your way into the DiGA directory

1. Conception
After the sparking idea, the conception begins, in which the target group, the benefit, the medical purpose and the requirements for your DiGA are defined.
UX and user research specialists

2. User Interface Design
A professional app design makes your app tangible for the first time and serves as an indispensable foundation for the implementation of the project. For visualization, we create wireframes and an initial click dummy, which is then filled with functionality.

3. MDR-compliant DiGA development
The regulatory jungle is obscure for many DiGA manufacturers at the beginning, because both the DiGAV and the MDR impose many requirements. In this step the functionalities of your DiGA are developed by us step by step, passing through our quality management processes according to ISO 13485 and IEC 62304.

4. Content Production
A medical app requires good content. Once the scientific evidence for the content is given, it is translated into multimedia formats. We support you with the creation of images, videos, texts and other visual elements for a unique user experience.

5. Implementation as a medical device
Your app will then be registered as a Class I, IIa or IIb medical device according to MDR. We will guide you through this process and assist you in finding a designated body if necessary.

6. BfArM approval
We support you with the application to the BfArM and prepare all documents. After the app has been tested by the BfArM with regard to safety, functionality, quality, data security and data protection (fast-track procedure), it is provisionally reimbursed by the statutory health insurance for a period of one year.

7. Inclusion in the DiGA directory
Now your DiGA will be listed in the BfArM directory and can be reimbursed by health insurers. We take care of regular software updates and the maintenance of your product.
Additionally, we offer advice on marketing your DiGA to reach more patients.
What really sets us apart

ISO 13485 certified
Our quality management system is certified according to ISO 13485. In this way, we ensure a compliant development of your DiGA and fulfill the requirements of the MDR for quality management systems.

ISO 27001 certified
QuickBird Medical is ISO 27001 certified for information security. We are experts in the areas of cybersecurity and health data protection.
Legal Manufacturer for your DiGA
If required, we can assume the role of legal manufacturer for your DiGA. We therefore bear legal responsibility for compliance with all regulatory requirements of the MDR. This allows you to focus on your core competencies such as sales & marketing.
QuickBird Medical receives first BSI TR-03161 certification
QuickBird Medical has developed a DiGA together with a customer. After intensive preparation, careful implementation and close cooperation with the testing body, it succeeded on June 2, 2025: The application was one of the first DiGA ever to receive the new BSI certificate in accordance with TR-03161, an important milestone for the entire DiGA scene.
QuickBird Medical receives first BSI TR-03161 certification
QuickBird Medical has developed a DiGA together with a customer. After intensive preparation, careful implementation and close cooperation with the testing body, it succeeded on June 2, 2025: The application was one of the first DiGA ever to receive the new BSI certificate in accordance with TR-03161, an important milestone for the entire DiGA scene.
Our Medical Software and DiGA customers
Are you planning to implement a DiGA?
Our team supports you in the planning and technical implementation of your application. In doing so, you benefit from our experience in implementing all regulatory requirements for DiGA. Contact us and arrange a no-obligation initial meeting. Let’s find out together how we can support you on your way to the DiGA directory.