What is the point of a DiGA if it does not improve patients’ lives? There is none!
This is why DiGAs are only justified when their positive supply effect has been proven. Only then do you have a chance of being permanently included in the BfArM register. Ultimately, your DiGA should deliver real added value for the patient. After all, health insurance companies only want to pay for measures that have a benefit.
In this article, we provide you with detailed guidelines on how to demonstrate positive supply effects.
Contents
- 1. What is a positive supply effect?
- 2. Required information in the application
- 3. Study to prove positive supply effects
- 4. Evaluation concept (for provisional admission)
- 5. Summary
1. What is a positive supply effect?
A positive supply effect can take many forms. We realize this, for example, when we look at a typical course of treatment for depression:
- Depression and other depressive symptoms
- First visit to the family doctor
- Making a suspected diagnosis
- Referral to a psychotherapist
- Waiting period of 6 months
- Diagnostics & diagnosis
- Outpatient psychotherapy (20 – 25 weeks)
- Relapse prophylaxis
Even this very simplified presentation clearly shows the areas in which patient care can be improved. This includes not only the optimization of therapy, but also the improvement of structural parameters, such as shortening waiting times or strengthening the patient’s role in therapy. The DVG basically divides the positive supply effect into two broad categories: medical benefits and patient-relevant structural and procedural improvements.
A DiGA must be certified as a medical device and therefore have a specific intended purpose. It should already be possible to derive the benefit assessment from this. Find out more about the intended purpose in this article: Formulating the intended purpose for (software) medical devices
1.1 Medical benefits
Basically, a medical benefit is any form of improvement in diagnostics and therapy. Specifically, the following four categories are defined as medical benefits:
- Improvement in the state of health
- e.g. reduction of symptoms
- Shortening the duration of illness
- e.g. faster rehabilitation after surgery
- Prolonging survival
- e.g. earlier detection of heart disease
- Improving the quality of life
- e.g. better handling of an illness
1.2 Patient-relevant structural and procedural improvements (pSVV)
As mentioned at the beginning, a DiGA does not necessarily have to support the treatment or diagnosis of a disease in order to achieve a positive care effect. Organizational improvements, such as shorter waiting times or easier access to medical services, are also seen as a positive effect on care according to the DVG. The pSVV is divided into the following aspects in accordance with DiGAV §8, paragraph 3:
- Coordination of treatment processes
- For example, a DiGA can optimize coordination between the patient and several service providers (e.g. psychotherapist and neurologist) in order to facilitate a better organized therapy process.
- Alignment of treatment with guidelines and recognized standards
- Implementation of patient guidelines in apps to show patients how they can participate in their own treatment. For example, an app could display health-related information and regularly remind users of necessary doctor’s appointments.
- Adherence
- Support in the implementation of measures agreed with the doctor. This is intended to encourage the patient to take an active role.
- Facilitating access to care
- DiGAs can help to improve access to medical care and thus compensate for barriers such as place of residence or other factors.
- Patient safety
- The focus here is on avoiding treatment errors. DiGA can prevent these or help patients to recognize errors themselves..
- Health literacy
- People often find it difficult to find and interpret health-related information. DiGA can transfer this knowledge in a targeted manner and enable patients to gain a greater understanding of their disease and therapy.
- Patient sovereignty
- DiGAs can encourage patients to participate in the decision-making processes in treatment and to share their experiences.
- Coping with illness-related difficulties in everyday life
- The aim here is to provide support in compensating for and reducing insecurities in everyday life. An application can ensure that a patient can adjust to a symptom at an early stage or support them in everyday activities.
- Reduction of therapy-related expenses and burdens for patients and their relatives
- Many therapies involve a great deal of effort on the part of the patient. An app can help here, for example, by shortening or sensibly bridging waiting times or minimizing physical presence at appointments.
If you look at the different areas, you quickly realize that pSVV is primarily about strengthening the role of the patient in their own care.
Economic aspects are deliberately not taken into account here. If your DiGA provides financial relief for patients and health insurance companies, this is a good argument for your price negotiations, but is not considered a positive effect of care.
QuickBird Medical Tip: Since a DiGA must be certified as a medical device, it must also have some form of diagnostic or therapeutic benefit according to the MDR. As a result, some applications that only cause a pSVV are not considered medical devices and therefore cannot become a DiGA.
Read more about the intended purpose according to MDR and the requirements for medical devices here.
2. Required information in the application
Regardless of whether you apply for permanent or provisional listing in the DiGA directory, some information is mandatory for all manufacturers.
2.1 Specification of the patient group
You must specify the patient group for which a positive care effect is to be achieved. Only a classification according to ICD codes is permitted. Each ICD code describes a diagnosis, whereby both three- and four-digit codes are permitted. Three-digit codes cover a broader patient group, whereas a four-digit code represents a more detailed specification of this patient group.

Meaning of ICD
Example:
- Three-digit code:
- F32 = Depressive episode
- Four-digit code:
- F32.0 = Mild depressive episode
- F32.1 = Moderate depressive episode
If your DiGA is to demonstrate a positive supply effect for several indications, you must provide evidence for each patient group. However, you can also prove the benefit for several indications at the same time if it is essentially comparable for these – but you must also include all indications in your study. It is up to the BfArM to decide whether the desired supply effect is then also present for all groups.
Caution: In the end, your DiGA can only be prescribed for the indications for which you can provide proof of benefit. For example, if you only define F32.1 as a patient group in your application, a doctor or psychotherapist can not prescribe the DiGA to patients who are diagnosed with F32.0 (mild depressive episode).
2.2 Indication of the positive supply effect
A DiGA must be able to demonstrate at least one positive supply effect. It is essential that this is also consistent with the purpose, functions and content of the DiGA and the published statements (e.g. in advertising material). You should therefore think about proving the positive supply effect at the same time as the medical device certification.
A purpose can no longer be changed so easily. So make sure that it is not formulated too specifically. You don’t want to waste the potential of your DiGA later on if it is also suitable for other indications, for example.
QuickBird Medical Tip: Proof of several positive care effects can create great added value for later price negotiations. Find out more about the price negotiations with the GKV-Spitzenverband here.
3. Study to prove positive supply effects
The proof of a positive supply effect provides the raison d’être for every DiGA. It goes without saying that this is one of the most important documents that must be submitted to the BfArM. There are clear guidelines for the study design, which we discuss in this chapter.
The study must be conducted in Germany. Countries in which the healthcare system is comparable to the German system are also conceivable – however, this involves a great deal of additional work and you will have to justify exactly why there is comparability here.
In addition, the study must also be entered in a public study register. In Germany, this is the German Clinical Trials Register (DRKS) at the DIMDI.
12 months after submission of the study results to the BfArM, the study must also be published. Publication on the website is sufficient.
Since you also have to conduct a study for certification as a medical device according to MDR in the course of the clinical evaluation, you should also consider aligning this directly with the requirements of the BfArM. If you proceed properly, you can use a study to conduct both the clinical trial and demonstrate the positive effect of the treatment.
QuickBird Medical Tip: We would like to advise you in advance to provide sufficient justification for the procedure in the study. For example, you must explain why you are using a particular study design or why the measurement methods used are suitable.
3.1 Permitted study types
A short expert interview with my family doctor should be enough as a study, right? No, it’s not as simple as that.
Applications in the DiGA directory are financed by health insurance companies and are used by patients across the board. The BfArM therefore has a strong interest in ensuring that meaningful findings are available for these products. That is why they only trust quantitative studies as is usual in clinical research. The minimum requirement is that it must be a comparative study. This is the only way to provide meaningful proof of benefit.
Simply put, you are comparing patients who use your DiGA with patients who do not use your DiGA.
Attention: It is not said that patients who do not use their DiGA do not receive treatment! The control group must be oriented towards the reality of care and receive the usual treatment for their situation. After all, you want to prove that your DiGA can improve the current situation.
Comparative study types can also be further subdivided. Examples include cohort studies, non-randomized and randomized controlled trials.
QuickBird Medical Tip: Randomized controlled trials have a very high validity compared to many other types of comparative studies. This makes your results less vulnerable. Officially, the choice of a meaningful study type does not bring any advantages on the part of the BfArM. Currently, however, almost every permanently listed DiGA in the directory has proven its positive treatment effects with a randomized controlled trial (as of 06/14/2021).
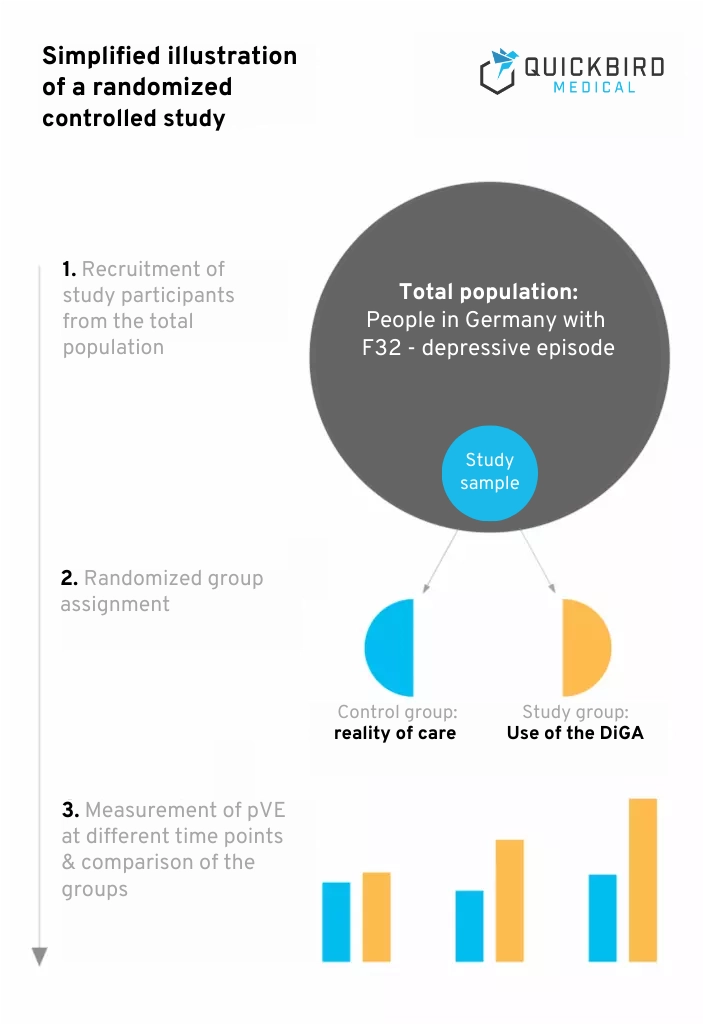
Simplified illustration of a randomized controlled study
In a controlled study, the participants are divided into groups. In the simplest case, this involves a control group and a study group. However, it is also conceivable to form several groups in order to be able to better classify the study results. Participants in the study group will use your DiGA in the study.
It is important that the control group is oriented towards the reality of care. If people with an indication usually receive a certain treatment, the participants in your control group should also receive this treatment. This is the only way to achieve a realistic benefit assessment. If people do not usually receive treatment (e.g. due to waiting times for a therapy place), study participants in the control group do not have to receive treatment either.
Example: Your DiGA is designed to support outpatient psychotherapy for depression by providing additional recommendations that support a patient in everyday life. Patients in this scenario therefore usually receive outpatient psychotherapy. You could therefore divide your study participants into the following groups:
- Group 1: Patients who only receive outpatient psychotherapy
- Group 2: Patients who receive outpatient psychotherapy & use the DiGA at the same time
As more and more DiGAs are being used across the board, the reality of care is also changing in some areas. If treatment with another DiGA is already a common procedure, you must also compare your DiGA in the study with the already established DiGA.
The reality of care that you have to depict in the control group can therefore take three forms:
- Non-treatment
- Treatment without the use of existing DiGA
- Treatment with the use of existing DiGA
An additional quality feature of studies is blinding. Blinding is intended to prevent study participants from knowing which group they are in. In drug trials, for example, a study group is often given a placebo preparation that has no medical effect. However, blinding is difficult to implement when examining apps.
3.2 Sample
The sample includes all participants that you include in your study. All these participants must have the diagnosis that you specify in your patient group.
A big question mark is often the sample size. In the course of case number planning, you must find out and justify which sample size is optimal for the study. An important aspect is the statistical methods used to investigate an endpoint. Many common methods for group comparison (e.g. variance analyses) have certain prerequisites, whereby, among other things, a certain group size should be achieved.
Samples that are too small are less representative and often lead to existing effects not being recognized.
When planning the number of cases, it is best to use studies already conducted in your field as a guide. If a pilot study is available, you can also derive findings for case number planning from it. The sample size is of course always dependent on the endpoints to be examined. The rarity of a disease also plays an important role.
For the study, it is also possible to use existing data (e.g. billing data from health insurance companies). This means that you do not have to form the control group yourself, but this is often associated with considerable effort and requires good argumentation with the BfArM.
You must also limit yourself to the endpoints that have already been measured and ensure that these have also been validly measured. They are therefore restricted in their freedom and have no influence on the quality of the data.
A before and after comparison is generally permissible. In this case, you collect data from the same people at different times – e.g. once before using the DiGA under conventional treatment and once after using the DiGA. However, here too, the treatment at the first measurement point must correspond to the reality of care. This procedure is mainly useful for stable conditions in which conventional treatment no longer achieves any improvement (e.g. diabetes).
3.3 Endpoints
The endpoints of a study describe its intended objectives. In principle, you can define as many endpoints as you like, but the primary endpoint will be the desired positive care effect – e.g. improvement in quality of life. The benefit rating can be measured using any number of endpoints.
The more endpoints you investigate and the more proof of benefit you can provide, the more advantageous this is for price negotiations. When defining endpoints, findings from the literature and the pilot study (if one exists) help.
Proof of benefit for a patient-relevant endpoint is sufficient for inclusion in the DiGA directory.
Even if they do not have to be completely congruent, endpoints must be consistent with the purpose of the application.
Important: Only patient-relevant endpoints count. Endpoints that do not represent a benefit for the patient do not play a role at the BfArM. These include economic aspects, for example.
Hypotheses
Every study must investigate specific hypotheses. These must be formulated in such a way that they can either be proven or refuted. Hypotheses are clear statements that are to be tested. Ideally, you should formulate a separate hypothesis for each endpoint for which you want to demonstrate an effect.
Examples :
- Patients who use DiGA have a higher quality of life after six months than patients who receive conventional treatment.
- Using DiGA shortens the duration of treatment compared to conventional therapy.
3.4 Method
Recognized methods must be used when measuring endpoints. In addition, endpoints are often only measurable through self-reporting by the study participants. In addition, endpoints are often only measurable through self-reporting by the study participants. The most important methods are medical measuring devices and psychological questionnaires.
Examples of measuring instruments for some positive supply effects:
- Quality of life:
- SF-36/SF-12
- SWLS – Satisfaction with Life Scale
- EuroQoL (EQ)-5D
- Overcoming difficulties in everyday life:
- SASS – Social Activity Self-Assessment Scale
- GAF – Global Assessment of Functioning
- Patient sovereignty/self-efficacy:
- ASKU – General expectation of self-efficacy
- SWOP-9 – Self-Efficacy-Optimism-Pessimism Questionnaire
If you use your own measurement methods, these must first be validated.
4. Evaluation concept (for provisional admission)
In the case of a provisional inclusion, the BfArM naturally wants at least an indication of why your DiGA can achieve a positive supply effect and how you plan to demonstrate this.
This is ensured in the form of an evaluation concept and a careful approach applies here. The concept must be prepared by a manufacturer-independent institution and comply with general scientific standards. It is important that the evaluation concept is suitable for demonstrating the positive care effect, as they must also adhere to this concept when conducting the study.
The scope of the evaluation concept is usually around 50 to 60 pages.
A good foundation stone for the evaluation concept is the mandatory “systematic user data evaluation”. This is based on data collected with the DiGA. Even if it is not mentioned publicly by the BfArM, this is to be understood as a pilot study.
This should not be underestimated! This means, among other things, that you need an ethics vote. A waiting period of several weeks or even months can be expected. When analyzing user data, your sample must also reach a certain size in order to have a certain significance (rule of thumb: 20-30 people).
Furthermore, a literature research is necessary for the evaluation concept. It should provide an overview of the current state of research in your field and give an indication of which endpoints are being investigated. The results of the user data evaluation should show that the DiGA works and can achieve the desired effect.
You are welcome to examine several endpoints in your pilot study. This will help you to plan the number of cases later and you can then decide which endpoints you would like to investigate in the planned study.
QuickBird Medical Tip: It also makes sense to combine this user data evaluation with the clinical evaluation according to MDR.
Other documents must also be attached to the evaluation concept. One document that the BfArM pays particular attention to is the Study protocol. In particular, logistical aspects of the study are defined here, such as financing, data management and responsibilities. Some information is duplicated in the evaluation concept and the study protocol. Make sure that all relevant information is also included in the study protocol.
A statistical analysis plan is obligatory. This includes information on randomization, blinding, endpoints, handling of missing data and statistical analysis.
QuickBird Medical Tip: Shortly before completion of the evaluation concept, we recommend an appointment with the BfArM in order to receive final suggestions for optimization.
4.1 Deadlines in the trial phase
With the notification of provisional inclusion in the DiGA directory, there is hardly any breathing space. This is when the 12 months start in which DiGA manufacturers have the opportunity to submit the required study results. During this period, the study must be fully conducted and evaluated in accordance with the evaluation concept.
Although it is legally possible to apply for an extension of the trial period, this must be plausibly justified and is not welcomed by the BfArM. In principle, the following applies: if the proof cannot be provided through the applicant’s own fault (e.g. due to incorrect planning), the application will be rejected. Only external factors are permitted, such as the coronavirus pandemic, which meant that the study could not be carried out as planned.
So do not speculate on an extension of this period right from the start – if this comes to the attention of the BfArM, the application for testing will also be rejected.
An extension must be applied for no later than three months after the expiry date. However, you should contact the BfArM immediately as soon as it becomes apparent that you will not be able to comply with them.
5. Summary
Proof of a positive supply effect is essential for permanent listing in the DiGA directory. The positive care effect is divided into two categories: medical benefit and patient-relevant structural and procedural improvement.
The positive care effect must be specified in the application together with the patient group (ICD code). Each manufacturer must prove the stated benefit for its DiGA in the course of an empirical study. The BfArM sets out a thick catalog of requirements for such studies. For example, the minimum requirement is that it must be a comparative study. Randomized controlled trials are particularly recommended.
Overview of the study requirements:
- There must be at least one comparative study
- The DiGA must be compared with the reality of care
- The study must demonstrate a positive difference in a patient-relevant endpoint
- Validated measuring instruments must be used to measure endpoints
- The study must be conducted in Germany
- The study must be entered in a public register
- All study results must be published in detail
Since the MDR also requires a study for the clinical evaluation of an app, it makes sense to align this with the requirements of the BfArM. This means you only have to carry out one study in total.
Applications for provisional inclusion in the DiGA directory must be accompanied by an evaluation concept that at least proves that the DiGA works and that there are indications of positive effects on care.
Contents of the evaluation concept (for provisional admission):
- Precise procedure for the study
- Systematic user data evaluation (pilot study)
- Literature research
- Study protocol
- Statistical analysis plan
QuickBird Medical takes over the development of your DiGA and accompanies you through to inclusion in the BfArM directory. Contact us at any time if you have any questions or would like to implement a specific project.
If you are planning a medical app, DiGA or DiPA and need support with the development, please feel free to contact us and we will discuss the whole thing together without obligation. We develop medical apps for companies and research institutions on a contract basis.
Further questions? Please feel free to call us (+49 (0) 89 54998380 or send us an e-mail (kontakt@quickbirdmedical.com).
We look forward to hearing from you!





