Telemedical communication platform for clear care structures for gene therapies


Summary
INTEGRATE-ATMP
Advanced therapy medicinal products (ATMPs) open up innovative possibilities for the treatment of rare and serious diseases. However, these therapies require special care and communication structures – which do not yet exist. This is a challenge that the ambitious INTEGRATE-ATMP lighthouse project is tackling over a four-year study period.
Thanks to funding of 13.6 million euros from the Innovation Fund of the Federal Joint Committee (G-BA), INTEGRATE-ATMP is paving the way for harmonized structures in patient care. Together with our partner Designit, we were selected from numerous applicants by the consortium management to develop the telemedicine platform together with the INTEGRATE-ATMP project team at Heidelberg University Hospital (UKHD).
The telemedical communication platform enables structured and direct communication between clinics, treating physicians and patients and thus facilitates and simplifies the direct exchange of information between all those involved in ATMP treatment (patients, physicians, case managers). The aim is to relieve patients in their everyday treatment and their treatment centers in their everyday care. A first version of the telemedical communication platform has now been developed.
Challenge
The biggest challenge is to efficiently combine medical data from a variety of different sources, including HIS, MARVIN-based registries, treating GPs, treatment centers and patients. It is essential to ensure flawless data linking, data robustness and data security.
These points are also essential for the scientific success of INTEGRATE-ATMP, which is why the project also required a tight schedule. The study participants are people who are actually ill, which is why the need for the software is based on the course of the disease in these patients. This requires development that is always accurate and targeted within the deadlines.
Process
During the study and development period, we are developing the patient app and the web dashboards for the clinics, doctors in private practice and a possible future data exchange with federal authorities in a collaborative process.
QuickBird Medical serves as the main contact for the consortium management at Heidelberg University Hospital, where the management and coordination of a broad consortium consisting of 24 German university hospitals, the Techniker Krankenkasse, patient representatives, professional societies, registry administrators and practicing physicians is based.
Regular meetings with technical teams and stakeholders create a constant framework for discussion. This enables us to review technical details as well as content-related and strategic issues. In this way, we ensured a consistent focus on users and their needs.
Result
The telemedicine platform developed by us is the first central system that seamlessly links the HIS, MARVIN-based registers, treating physicians, treatment centers and patients. It establishes itself as a central interface in a previously fragmented healthcare system.
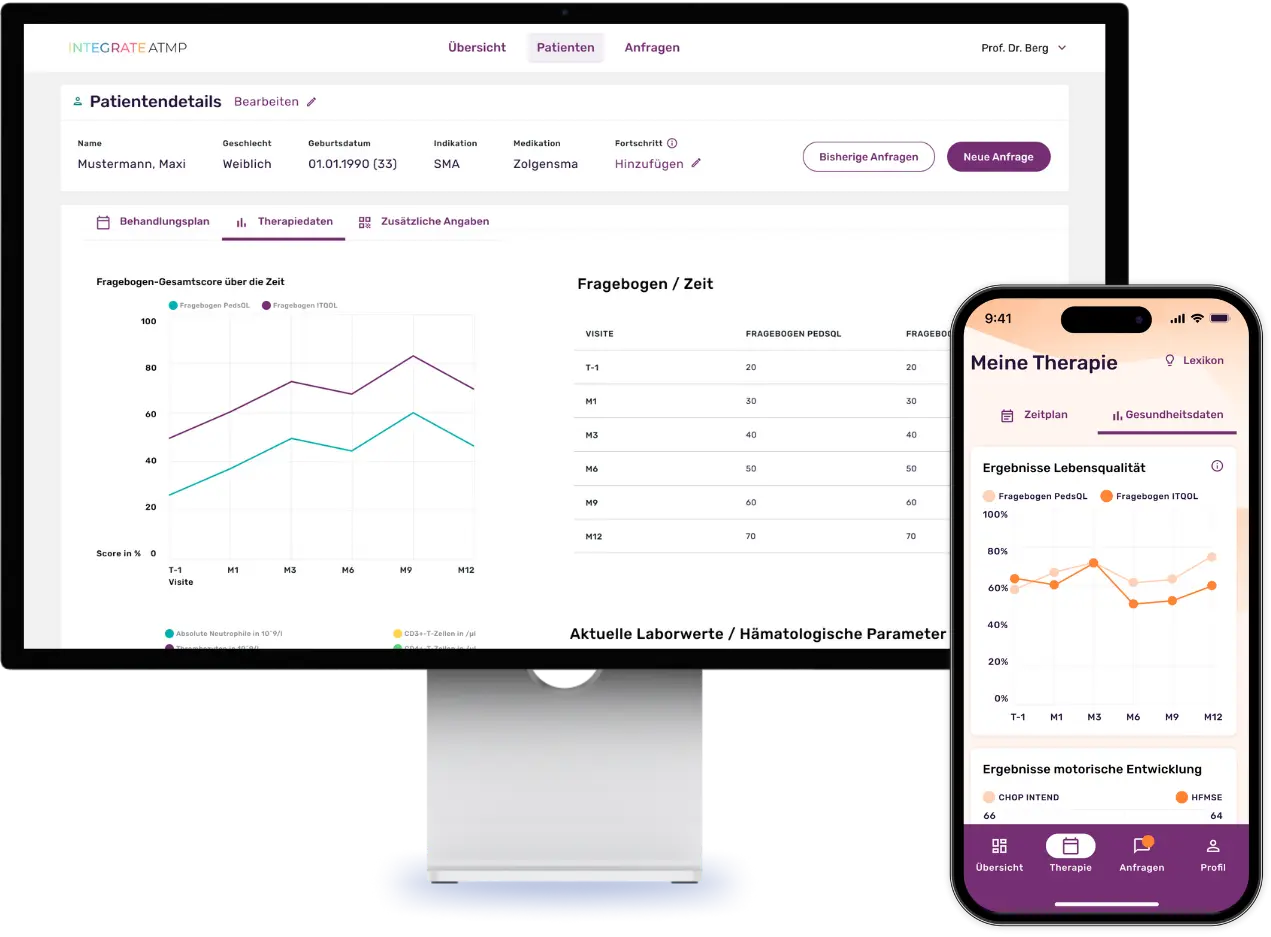
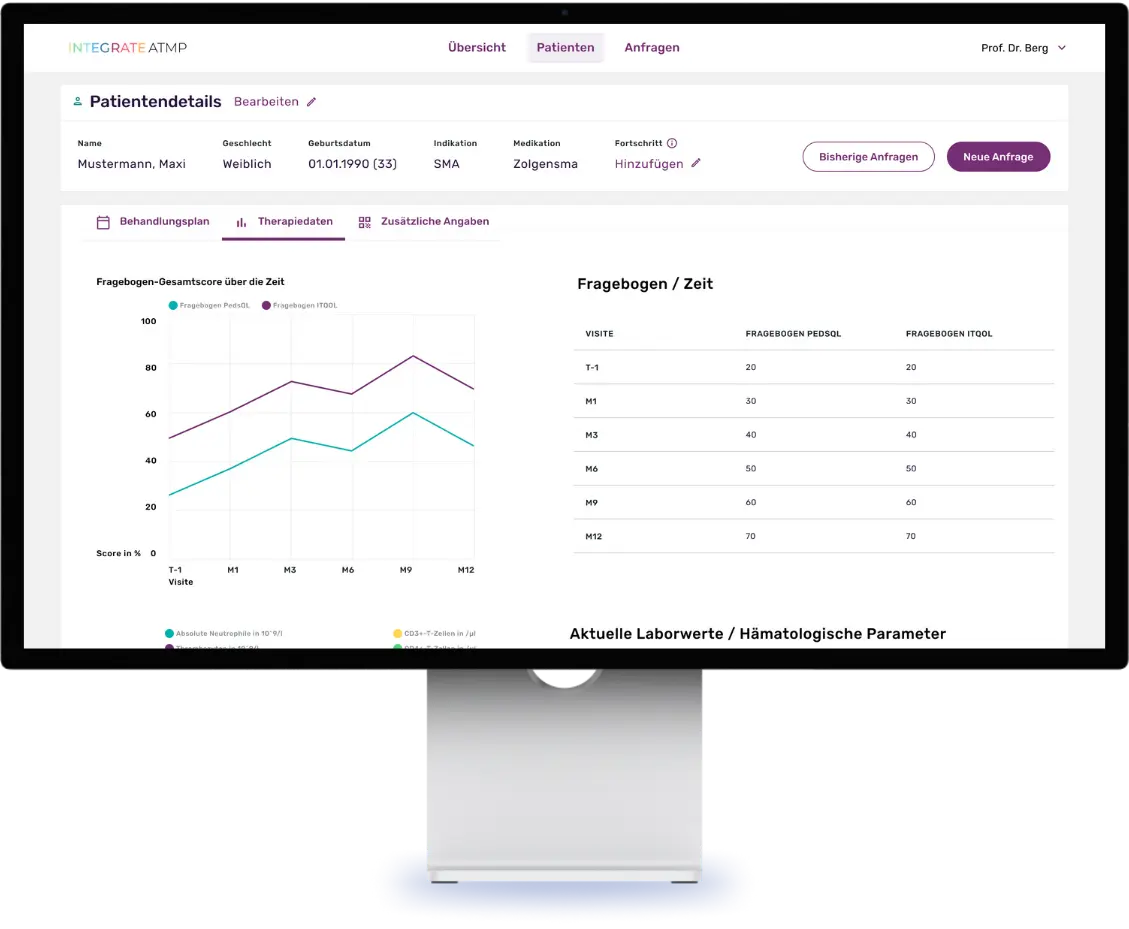
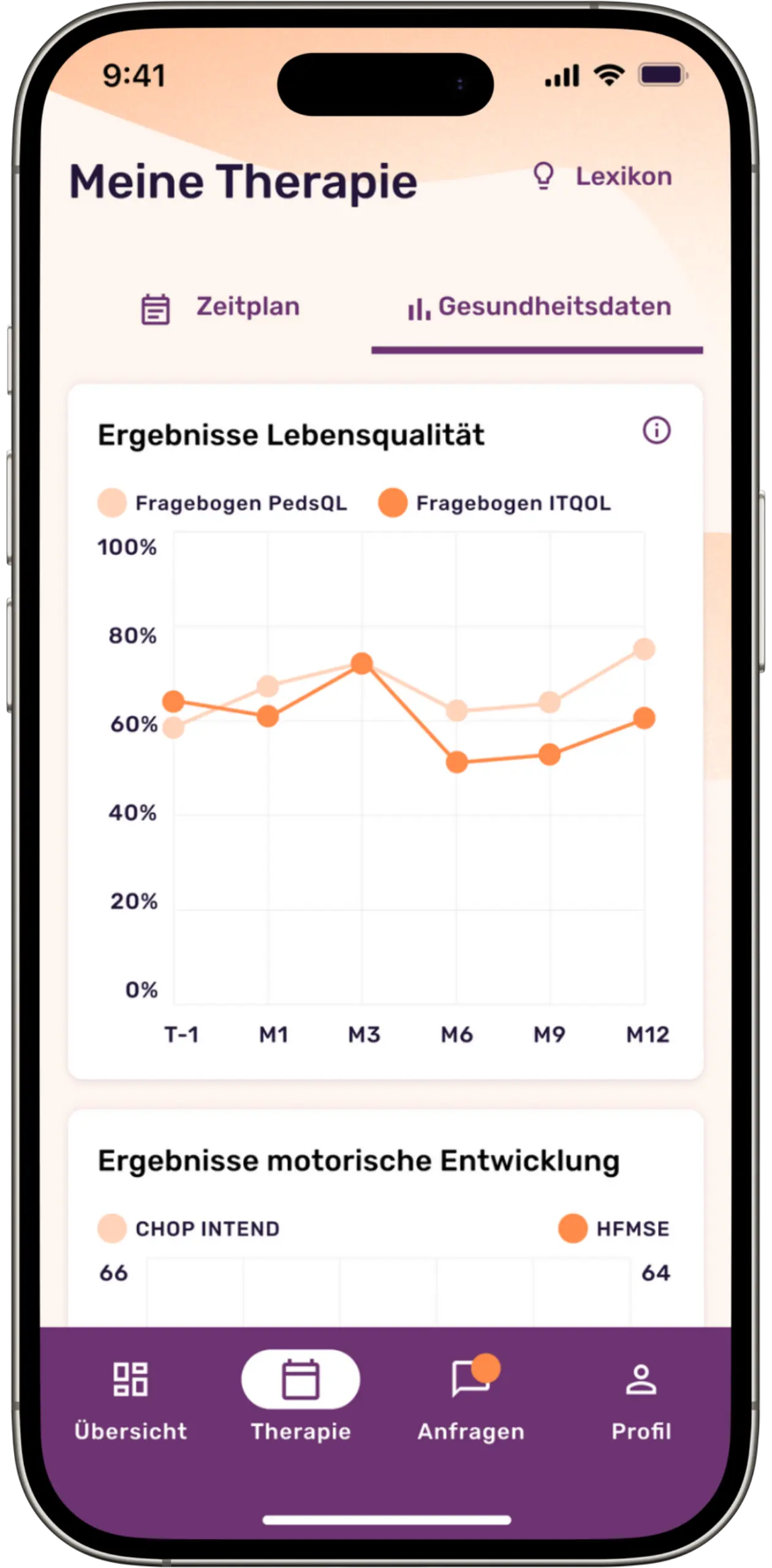
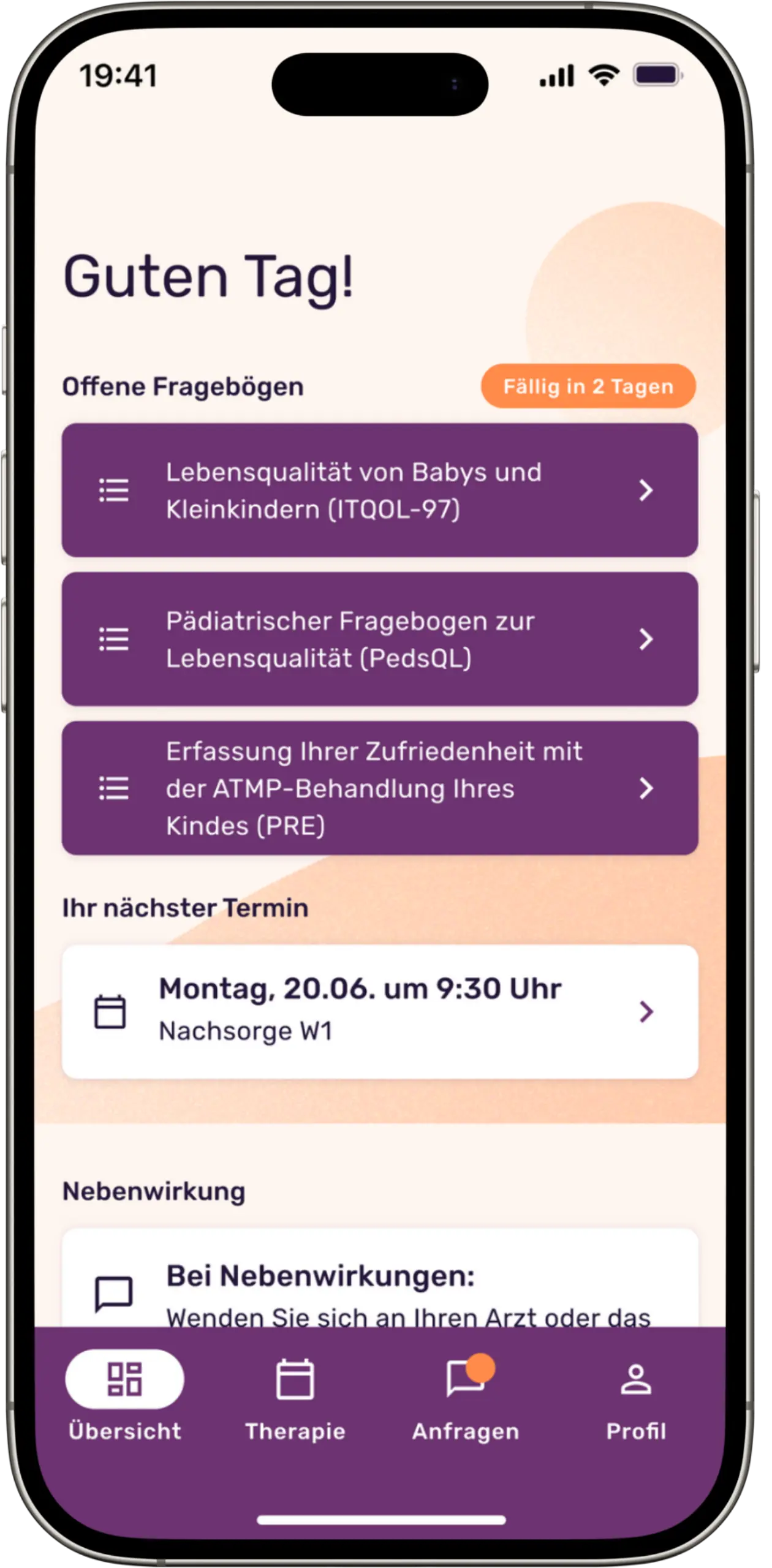
Our platform makes it possible to systematically record patients’ individually perceived quality of life by digitizing scientific questionnaires and making them usable for queries. Thanks to the automatic data collection via these digital questionnaires, the quality of the study results is increased in the best case and the data collection effort is significantly minimized. In addition, the platform facilitates constant contact between doctors and patients through a chat function, enables the sharing of relevant files and centralizes disease-specific documents and laboratory values.
A special feature of the platform is the integrated expert panel for side effect management for a subgroup of ATMP patients: When treatment problems arise, this is convened and provides treatment recommendations based on a holistic approach by experts on the platform.
Screenshots

Future
The main objective during the four-year study phase in INTEGRATE-ATMP is to make a decisive contribution to optimizing the treatment of rare diseases with ATMPs and to relieve clinics and treating physicians in their daily treatment routine by establishing standardized outpatient treatments and, for the first time, a direct communication channel on a nationwide level. The ATMP register being created as part of the project is intended to simplify the collection of data for future ATMP approvals.