On December 19, 2019, the Digital Care Act (DVG) made it possible for doctors to prescribe digital health applications (DiGA) to patients in a standardized way for the first time.
With the Digital Care and Nursing Modernization Act (DVPMG), a counterpart to this was also created for nursing care in June 2021: digital nursing applications (DiPA).
DiPAs are designed to support the everyday lives of people in need of care and their environment through web and smartphone apps. For example, a digital care application could help to minimize the risk of falls for people in need of care or improve communication between relatives and carers.
The Regulation on the Assessment of the Reimbursability of Digital Care Applications (DiPAV) provides information on how the DiPA application process works and what requirements DiPA must meet in order to be included in the directory of digital care applications.
In this article, we explain the difference between DiPA and DiGA, discuss the most important aspects of DiPAV, and ultimately provide a guide to the DiPA procedure. This guide outlines the process your application must go through in order to be included in the BfArM directory.
Table of contents
- 1. Latest Updates: DiPA in the Act on the Expansion of Powers and Debureaucratization in Care (BEEP)
- 2. Distinction between DiPA and DiGA
- 3. Guidelines for DiPAV
- 4. Further ways to achieve reimbursement by health insurance companies
- 5. Conclusion
1. Latest Updates: DiPA in the Act on the Expansion of Powers and Debureaucratization in Care (BEEP)
Important: In this article, we describe the current legal regulations governing DiPA. However, in November 2025, the BEEP Act (Act on the Expansion of Powers and Debureaucratization in Care) was passed by the German Bundestag, which in its current version provides for relevant changes with regard to DiPA.
The BEEP Act was supposed to come into force on January 1, 2026, but was referred to the Mediation Committee by the Federal Council due to last-minute amendments relating to hospital financing. If it comes into force unchanged, it would contain important improvements for DiPA:
- A trial year for DiPA is to be introduced in line with the DiGA fast track. This is conditional on an appropriate evaluation concept, as full proof of benefit is not yet available. At the same time, the BfArM is removing the requirement for supplementary support services to undergo a necessity assessment.
- Remuneration negotiations with the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds) can run in parallel. The entitlement to care begins as soon as the remuneration has been agreed. This then applies retroactively from the date of inclusion in the directory.
- The entitlement to care for persons in need of care will be increased from the current €50 per month to €70. Of this, €40 will be allocated to the DiPA itself, and €30 may be reimbursed for supplementary support services if necessary. However, it should be noted that this monthly budget may already be used up when a single DiPA is used. However, since several DiPAs can theoretically be used in parallel, this regulation could still mean that only the first application is reimbursed and any further DiPAs must be paid for out of pocket by the caregivers or those receiving care.
- The focus is on supporting caregivers: DiPA manufacturers are no longer required to provide evidence of a nursing benefit for the person in need of care. If DiPA achieves a reduction in the workload for caregivers, it is assumed that this will have a positive effect on those in need of care. To prove this relief, an evaluation must be submitted. How this should look is not defined in detail.
- In the future, DiPA will not only provide relief, but also contribute specifically to preventing the condition of people in need of care from deteriorating further, i.e., they will have a preventive effect and stabilize existing independence.
- The use of DiPA remains limited to outpatient home care; its use in inpatient care is not planned.
Note: As the BEEP Act has not yet come into force and changes to its content are still possible, we will update this article as soon as new information becomes available. Below, we provide information about the current legal regulations governing DiPA.
2. Distinction between DiPA and DiGA
To better understand the digital care application, it helps to differentiate its use and content from the DiGA.
DiGA are apps that support sick people in the treatment, diagnosis, compensation or alleviation of illnesses, injuries and disabilities. Their use was recognized on December 19, 2019 with the DVG legally established. Since then, DiGA can be reimbursed by health insurance companies as part of medical and psychotherapeutic treatment. DiPA is a similar concept, but is primarily aimed at people in need of care. It was incorporated in mid-2021 through the Digital Care and Nursing Modernization Act (DVPMG) into the eleventh German Social Code (SGB).
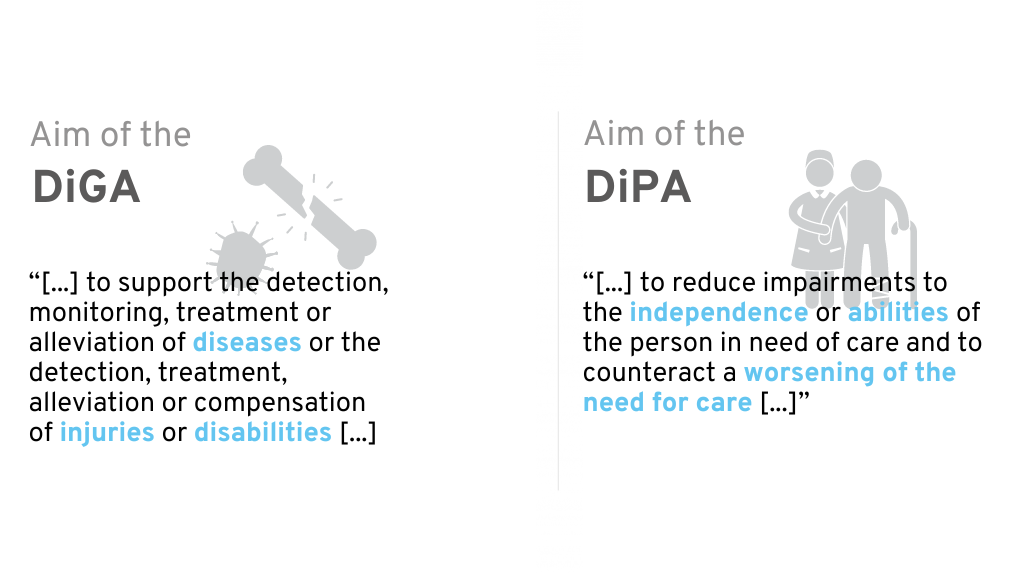
Distinction between DiGA and DiPA
As the distinction between DiGA and DiPA is not entirely obvious at first glance, we would like to highlight the most important differences in the following chapters.
2.1 Health insurance vs. social care insurance
The first major difference between DiGA and DiPA can be found in their place in the legal text. DiGA are defined in SGB V – Statutory Health Insurance, DiPA in SGB XI – Social Care Insurance. A DiGA is therefore reimbursed by the health insurance fund, whereas the costs of a DiPA are covered by the social care insurance fund.
A DiGA is prescribed by doctors and psychotherapists and is explicitly intended to support people with specific indications in terms of relief, therapy, and diagnosis. Similar to medication, the prerequisite for reimbursement of a DiGA by health insurance is a prescription from a doctor or psychotherapist. Direct approval from the health insurance company is also possible. A digital care application, on the other hand, can only be applied for by those in need of care from their long-term care insurance provider.
End user application for a DiPA – Procedure
The provision of a DiPA is approved by the long-term care insurance fund upon application by the person in need of care. The initial approval must be limited in time and may not exceed six months. Within this period, the long-term care insurance fund checks whether the digital care application has actually been used and whether the purpose of the DiPA is being achieved in the specific care situation.
If this review shows that both requirements are met, a permanent permit shall be issued. A new application is not required for this.
The permanent approval means that the nursing care insurance fund will grant the monthly reimbursement of up to €50 on a permanent basis, as long as the legal eligibility requirements are met.
2.2 A DiPA does not have to be a medical device
40a Digital care applications SGB XI:“(1) People in need of care are entitled to care with applications that are essentially based on digital technologies” (Translated from German)
33a Digital health applications SGB V: “(1) Insured persons are entitled to care with low-risk class medical devices whose main function is essentially based on digital technologies […].” (Translated from German)
These are the introductory words of both paragraphs in the legal text, which immediately make it clear that there is a big difference in terms of the requirements for the digital care application.
While DiGA are explicitly defined as class I or IIa medical devices, DiPA only refer to “applications”. A DiPA may or may not be a medical device. This means that many of the regulatory requirements with which DiGA manufacturers struggle are (at first glance) not relevant for many DiPAs.
However, the BfArM also places similar requirements on DiPA that are not medical devices. For example, manufacturers must have a quality management system in place and meet requirements for the safety and functionality of the app. In the current DiPA guidelines (as of October 11, 2023) therefore reference numerous ISO/IEC standards for medical devices and refer to the Medical Device Regulation (MDR) in many places. As a result, the requirements for DiPA do not differ significantly in most cases, as they are based on the requirements for medical devices according to MDR.
If it is a medical device, it must belong to a low risk class (I or IIa).
If your application is to be certified as a medical device, you can find further information here.
2.3 Price cap
While the price limit for DiGA is based on other applications in the same category, a fixed price cap is planned for digital care applications. This means that a maximum of €50 per month can be reimbursed for a DiPA ( §40b SGB XI). Although this has been the subject of heated debate among experts, the law is clear. In principle, a DiPA can also be more expensive, but any additional costs must then be borne by the consumer of the DiPA.
2.4 Can a DiPA also be a DiGA?
In principle, an application can be both a DiPA and a DiGA. The current status for manufacturers is that both applications can be submitted for one and the same app. However, deviations in the requirements must be noted. If a listing in both directories is achieved, the consumer’s service provider must check individually which health insurance fund will reimburse the application.
3. Guidelines for DiPAV
We have the contents of the DiPAV summarized in the form of a guideline, which clearly explains the application as well as the requirements and their proof. Particularly important is the Proof of the nursing benefits, which is also explained.
3.1 Requirements for DiPA
The requirements are listed in the legal text at an abstract level. Central assistance is provided by the Appendices 1 and 2 of the DiPAV, as well as Appendix 1 of the DiGAV, as these contain checklists that manufacturers can use to check the individual requirements for their product.
The following requirements apply to DiPAs:
- Safety functionality (see DiPAV Annex 1): A distinction is made between medical devices and non-medical devices. Medical devices fulfill the requirements for safety and functionality through the CE conformity marking. Non-medical devices must fulfill the requirements according to Annex 1 of the DiPAV, which are very similar to the medical device requirements for risk class I, however. This requires, for example, the establishment of a quality management system for medical devices (e.g. in accordance with ISO 13485), as well as a risk management system.)
- Data protection and data security: In addition to the GDPR and the general state of the art in data security, the requirements specified by the BSI and the data protection test criteria specified by the BfArM for DiGA and DiPA must be met. In addition, all requirements from Appendix 1 of DiGAV must also be met for DiPA. However, as some points are not directly transferable, the BfArM’s DiPA guidelines provide further information on how to deal with them.
- Quality requirements: (see DiPAV Annex 2)
- Interoperability: Similar provisions apply here as for DiGA. An interoperable export of data must be offered in both machine-readable and human-readable/printable form. The DiPA must also allow medical devices used by users to be connected if data from them is required.
- Robustness
- Consumer protection
- Age-appropriate usability, user-friendliness and accessibility
- Support for people in need of care and users
- Quality of care-related content
- Safety of those in need of care (patient safety)
- Nursing benefits
What is a nursing benefit?
The nursing benefit of a DiPA exists if the use of the application prevents an impairment of independence or abilities of the person in need of care is counteracted. This also includes the progression of a person’s need for care.
In addition, there must be a nursing benefit in at least one of the following areas:
- Mobility
- cognitive and communicative skills
- Behavioral and psychological problems
- Self-catering
- Coping with and independently dealing with illness- or therapy-related demands and stresses
- Organization of everyday life and social contacts
→ In addition to these areas, the care benefit can also be given in the area of household management.
It is important to note that DiPA users do not necessarily have to be the person in need of care themselves. Even if If caregivers are supported in providing assistance in one of the above-mentioned areas through the application, there is a nursing benefit.
Admissible studies to prove the nursing benefit
In order to demonstrate the nursing benefits, manufacturers must submit comparative studies. These may also be retrospective comparative studies, including retrospective studies with intra-individual comparisons.
Alternatively, prospective studies, and specifically randomized controlled trials, are recommended due to their high informative value. The studies should be conducted in Germany. If this is not possible, transferability to the German healthcare context must be demonstrated.
Unfortunately, the only publicly known application for listing in the DiPA directory 2024 was rejected precisely because of the study. According to Lindera’s press release, the BfArM criticized, among other things, that the study submitted was unable to prove the effects of the app in isolation from existing care services.
3.2 Application
Application procedure
The application for inclusion in the DiPA directory is submitted by the manufacturer to the Federal Institute for Drugs and Medical Devices (BfArM).
14 days after receipt of the complete application documents, the BfArM confirms receipt of the documents to the applicant or requests missing documents. The manufacturer has up to three months to complete missing documents. If no complete application documents have been submitted by the end of this period, the application will be rejected.
Once all documents are complete, the BfArM normally has 3 months to review the application and come to a decision regarding inclusion in the DiPA directory.
Content of the application
It is important to note that digital care applications can only be listed permanently. There is no trial phase. In plain language, this means that all necessary documents – including proof of the nursing benefits – must already be available at the time of application. The application includes:
- Electronic application via the BfArM application portal
- Documents proving the nursing benefit
- Documents to prove compliance with all requirements
- Any additional documentation (e.g., instructions for use or, in the case of medical devices, conformity assessment according to MDR or MDD)
The manufacturer must also provide the following information in the application (according to §2 DiPAV):
- Manufacturer and identifying features of the DiPA
- The intended purpose
- Associated notified body (if applicable)
- Certificates issued by the notified body and the manufacturer’s declaration of conformity (if applicable)
- Instructions for use
- Purpose, mode of operation, content and use of the DiPA in a generally understandable form
- DiPA functions
- Institutions and organizations involved in the development of the DiPA (if applicable)
- Sources for the nursing-related content and procedures implemented in the DiPA, in particular nursing-medical guidelines and expert standards, textbooks and studies
- Evidence of a nursing benefit including supplementary support services provided by third parties (according to “Evidence of nursing benefit” and “Studies to demonstrate nursing benefit”) in a simple and generally understandable summary
- Group of people in need of care for whom a nursing benefit has been proven
- Care benefits that have been demonstrated for the specified group of care recipients and users
- Study/studies by the manufacturer to demonstrate the nursing benefits including supplementary support services by third parties
- The fulfillment of the requirements and specifications for a DiPA including the respective questionnaires
- Intended user roles
- Quality-assured use of DiPA in the home environment, including exclusion criteria for use
- The type, content, scope and duration of the third-party support services required for the use of DiPA, if applicable
- Data processing locations
- Manufacturer’s compatibility commitments regarding supported platforms and devices as well as required accessories and other product components (if applicable)
- Standards and profiles used as well as human-readable export formats to create semantic, syntactic and technical interoperability
- Sum insured under the liability insurance taken out by the manufacturer for personal injury
3.3 Consultation from the BfArM
The BfArM advises manufacturers both before and after inclusion in the DiPA directory. The topics covered in the consultations are largely determined by you as the manufacturer. It is possible to clarify general questions of understanding regarding the DiPA requirements or to discuss product-specific documents.
For manufacturers whose DiPA is already listed, however, questions relating to the modification of the product can be dealt with.
The consulting fee ranges from €250 to €5,000 (admittedly a very wide range).
3.4 My DiPA is listed! What now?
After a positive decision by the BfArM, your DiPA will be included in the directory. Congratulations – at this point you have completed a large part of the work and can celebrate with your team. However, you are not quite there yet: within three months of the listing, the remuneration amount for the DiPA will be negotiated with the National Association of Nursing Funds. In addition, technical and contractual framework conditions for the provision of the DiPA must be regulated in accordance with Section 40a (4) SGB VI.
3.5 Arbitration proceedings
The price for the DiPA is negotiated during the first three months negotiated between the manufacturer and the nursing care insurance fund after inclusion in the BfArM directory. If these two parties cannot reach an agreement, the arbitration tribunal determines the price of the DiPA. Details on the arbitration procedure can be found in section 8 (§ 30 – § 32) of the DiPAV.
4. Further ways to achieve reimbursement by health insurance companies
In addition to the DiPA procedure, there are also several other ways in which software manufacturers can obtain reimbursement under the German healthcare system. These include, among others:
-
- Overview of all paths to reimbursement: Read our white paper
- Reimbursement for software in hospitals: Read our white paper
- Digital health applications – DiGA procedure: To our guide
- Selective contracts with health insurance companies: Our guide
- Reimbursement for software in the GKV medical aids directory (HMV): See our guide
- Central Prevention Review Board (ZPP): About our guidelines
- NUB as cost reimbursement for software products in hospitals: See our guide
In addition to the aforementioned reimbursement options, it is also worth looking into public funding opportunities that can bridge the gap. First and foremost, it is worth taking a look at the G-BA’s innovation fund, which provides financial support for digital healthcare solutions in their early stages.
You can find more information in our guide to the Innovation Fund: Go to guide
5. Conclusion
It is not easy to meet all of the DiPA requirements. In particular, submitting studies as proof of effectiveness at the time of the initial application is a challenge for manufacturers. There is a fast-track procedure for DiGA approval, which allows manufacturers to demonstrate the benefits of an application in a study within one year. However, such a procedure has not yet been implemented for DiPA. It remains to be seen whether the fast-track mechanism will be enshrined in law in the near future as planned with the BEEP Act.
This guide is regularly revised to ensure that it is up to date.
QuickBird Medical develops health apps and medical software for companies and research institutions on a contract basis. If you are planning a medical app, DiGA, or DiPA and need support with development, please contact us and we will discuss the project with you without obligation.




