Digital Therapy for the Treatment of an Overactive Bladder
Summary
kontina
kontina is a medical device for the treatment of symptoms of an overactive bladder (OAB).
The app helps users to reduce symptoms themselves, promote their own bladder health in the long term and thus regain a better quality of life.
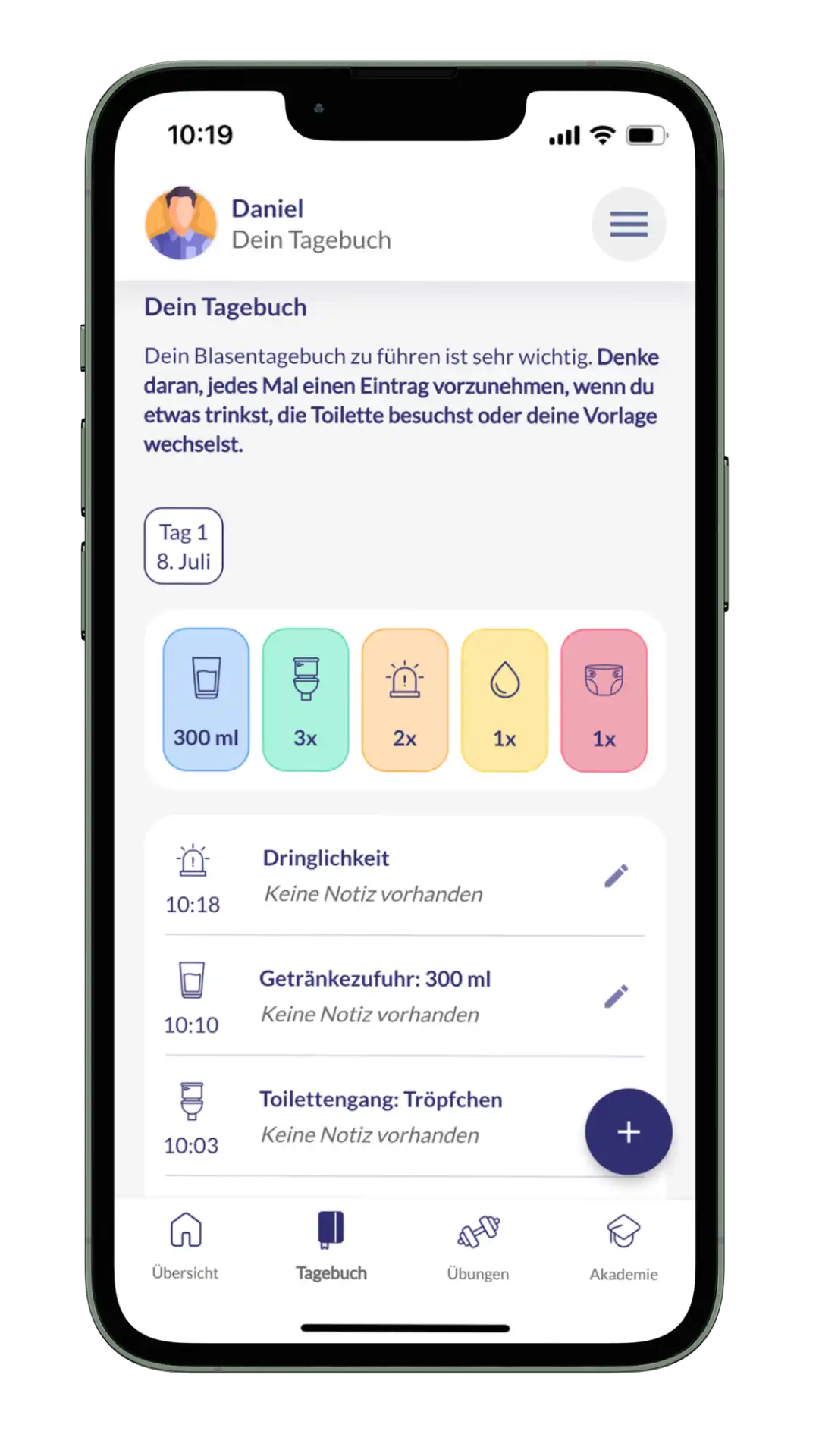
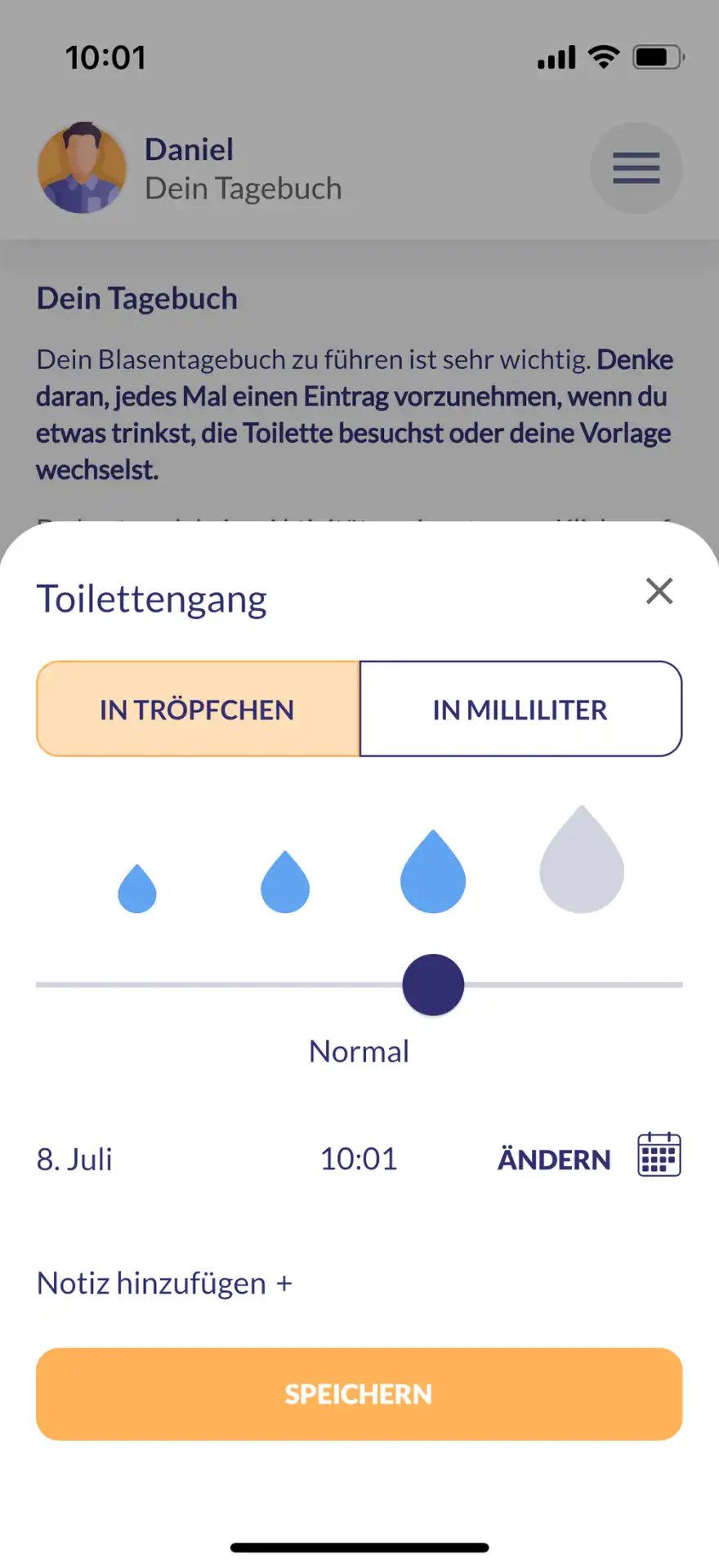
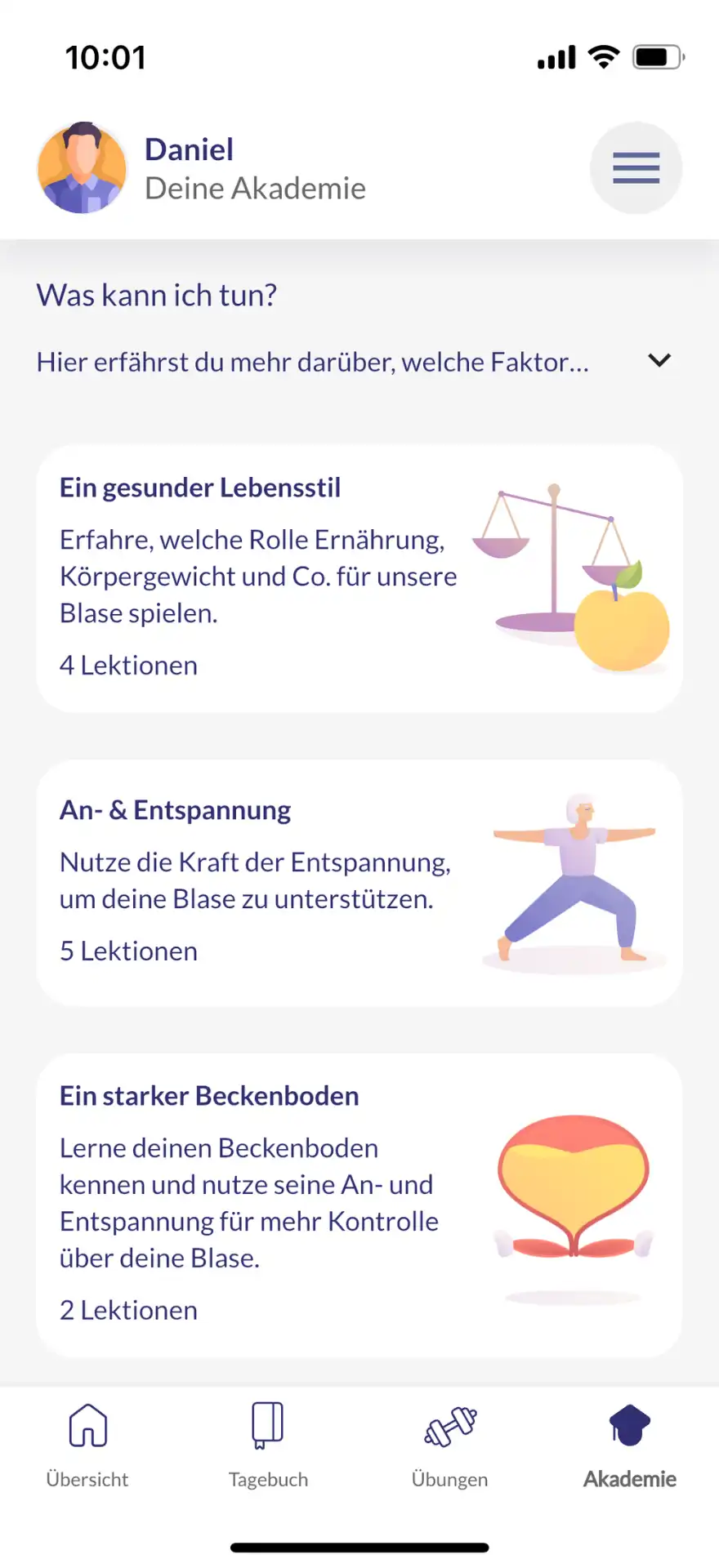
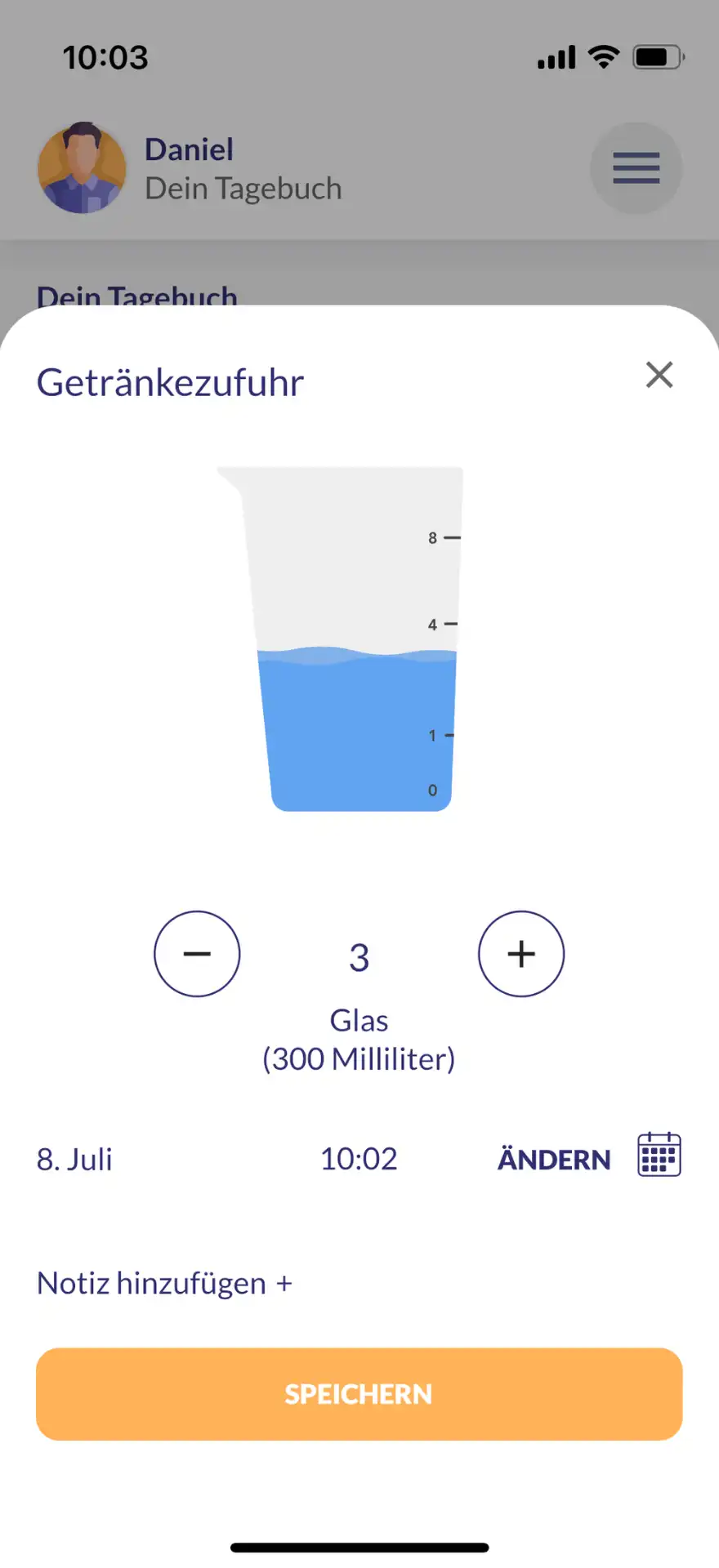
The main functions of kontina include holistic exercises for bladder and pelvic floor training, interactive learning content, practices for acute help with urge waves and a bladder diary in which users can document their progress and thus obtain an overview of the course of therapy.
Challenge
QuickBird Medical has launched the app on the market as a so-called legal manufacturer. We therefore assume full regulatory responsibility for the MDR medical device. This allows our customer to focus entirely on its own core competencies in the areas of science, marketing, sales and user support. In addition, compliance with the strict data security standards in accordance with BSI TR-03161 was a challenging task that had the highest priority in order to ensure the protection of sensitive data.
Process
Our ISO 13485-certified quality management system is used for the agile further development of the application. In addition, we have implemented comprehensive data security measures as part of our ISO 27001-certified information security management system (ISMS) to ensure reliable protection of all processed data.
Result
kontina is a certified class I medical device in accordance with the European Medical Device Regulation (MDR), which meets all the necessary quality and safety standards. In addition, compliance with all safety requirements has been successfully demonstrated: An external security penetration tester has comprehensively tested the product and confirmed the effectiveness of the implemented security precautions.
Screenshots
Future
kontina is a CE-certified medical app. We continue to support the product in order to fulfill our obligations as a medical device manufacturer and to ensure patient safety and performance. Our software team is also responsible for the maintenance, operation and further development of the software.