The Medical Device Regulation (MDR) poses many challenges for manufacturers of software medical devices. In addition to establishing a quality management system (QMS) and preparing technical documentation, manufacturers face regulatory liability risks.
For many companies, this raises the question:
Is there a way to completely outsource the regulatory obligations for medical device manufacturers to a service provider?
Yes, there is: outsourcing the role of legal manufacturer to an external company. This is an efficient and risk-minimizing solution that conserves your resources while ensuring regulatory compliance.
In this guide …
- … we highlight the advantages of an external legal manufacturer for your product,
- … we answer the most important questions about this approach and
- … we take a look at which companies this model really makes sense for.
Table of Contents
- 1. Problem: Obligations for legal manufacturers of medical devices
- 2. Solution: External distributor for MDR medical devices
- 3. Advantages of an external manufacturer according to MDR
- 4. Procedure for such cooperation to outsource the role of legal manufacturer
- 5. Important questions and answers
- 5.1. I would like to outsource the manufacturing role in order to bring the product to market more quickly. However, I would like to take over the manufacturing role for the product again at a later date. Is this possible?
- 5.2. Do I retain all ownership rights to the product?
- 5.3. Is that more expensive than handling the topics in-house?
- 5.4. Who is liable for the product in a regulatory sense, e.g., in the event of patient injury?
- 5.5. Can the manufacturer role for a DiGA be outsourced?
- 5.6. Can I subsequently outsource my manufacturer role for a certified medical device?
- 5.7. Can I further develop the product after it has been placed on the market?
- 5.8. Can I continue to use my own development team?
- 6. Examples of medical device manufacturers with external distributors
- 7. Conclusion: Who benefits from outsourcing manufacturing?
1. Problem: Obligations for legal manufacturers of medical devices
If you want to develop software that is to be certified as a medical device, you will face considerable effort. According to MDR, the manufacturer of a software medical device must fulfill the following obligations, among others:
- Establishment of a quality management system (QMS): Establishment of processes and structures (according to MDR & ISO 13485)
- Preparation of technical documentation: Compliance with MDR, IEC 62304, IEC 62366-1, ISO 13485, IEC 82304, ISO 14971 (to name only the most important standards and laws here)
- Liability for product risk under the MDR: As a manufacturer, you are liable for patient harm or breaches of your regulatory obligations. In the event of negligent conduct, you may even be personally liable under criminal law.
- Continuous implementation of all quality management processes: post-market surveillance (PMS), post-market clinical follow-up (PMCF), vigilance, management reviews, internal audits, training, computerized systems validation (CSV), risk management, clinical evaluation, etc.
- Monitoring information security: You must have control over the information security of your product and your company through structured processes and measures.
2. Solution: External distributor for MDR medical devices
If you do not wish to or are unable to fulfill the above obligations, there is a sensible alternative: outsourcing the manufacturer’s role under MDR to a service provider.
This allows you to focus on your core competencies while the service provider assumes full responsibility for the regulatory launch of your software medical device within the framework of the MDR.
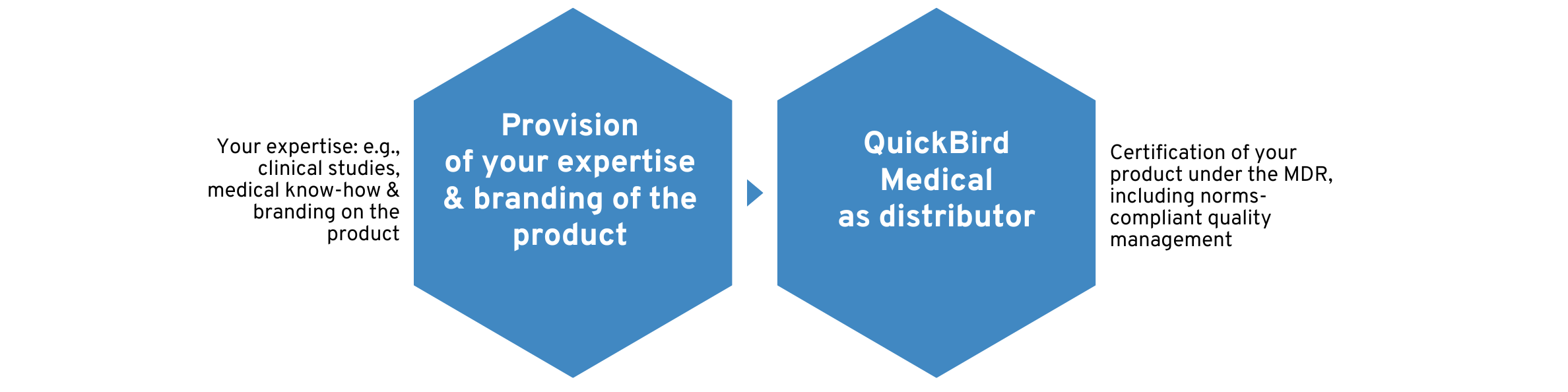
QuickBird Medical as an external distributor for your software product
Note: In this article, the terms ‘legal manufacturer’ and ‘manufacturer’ are used interchangeably.
2.1. Distributors and manufacturers for your software medical device
Different regulatory roles arise depending on the distribution of responsibilities. A common distribution of roles is that QuickBird Medical assumes the role of the so-called legal manufacturer according to MDR and you assume the role of the so-called distributor.
Accordingly, QuickBird Medical is liable for all obligations under the MDR that apply to medical device manufacturers.
You can then place your own logo/design/branding on the product, but successfully outsource the regulatory work.
As a distributor, you may then be responsible for customer support, sales, and marketing of the product. Here, too, there are certain obligations that you must observe, for example, under the German Medicinal Products Advertising Act (Heilmittelwerbegesetz). We will train you in these areas and provide you with support. This reduces your workload in relation to regulatory issues to an absolute minimum.
2.2. OEM and PLM for your software medical device
Under the previous Medical Device Directive (MDD), the so-called OEM-PLM constellation was common:
- OEM (Original Equipment Manufacturer): The OEM is the actual developer and manufacturer of the software medical device. It creates the complete technical documentation, carries out all necessary conformity assessment procedures, and initially brings the product to market in compliance with the law. The OEM therefore bears regulatory responsibility for product safety and conformity.
- PLM (Private Label Manufacturer): The PLM takes the finished medical device from the OEM and markets it under its own name, logo, and branding.
Following the replacement of the MDD by the MDR, the OEM-PLM constellation no longer exists in its original form. Instead, a distributor-manufacturer constellation is referred to as an alternative, which is described in the previous chapters. This means that the same objectives can still be achieved under the MDR.
3. Advantages of an external manufacturer according to MDR
Outsourcing the role of manufacturer to an external legal manufacturer offers the following advantages, among others:
- Faster and easier market access: An external manufacturer enables products to be brought to market much faster, as it has existing certifications (e.g., ISO 13485) and proven quality management processes in place. This saves time and avoids delays caused by time-consuming internal preparations.
- Reduction in resources and costs: Regulatory obligations are completely outsourced, allowing companies to free up internal resources such as personnel and financial resources for other projects. The high costs of setting up and maintaining a QMS, certifications, and audits are eliminated or significantly reduced.
- Regulatory certainty and risk minimization: The external manufacturer brings comprehensive regulatory expertise to the table and ensures that products comply with the latest regulatory requirements. The risk of liability in the event of product recalls or safety incidents is also outsourced. In addition, the external manufacturer remains responsible for adjustments to new regulations, which minimizes uncertainty for the company.
- Flexibility and focus on core competencies: Companies can concentrate on their strengths, such as product development and innovation, without being distracted by regulatory requirements. At the same time, an external manufacturer offers flexibility in the organizational structure by taking on regulatory obligations.
- Strategic advantages: By working with an external manufacturer, companies gain access to industry-specific networks and proven processes that facilitate market entry. In addition, there is no need to build up and maintain regulatory knowledge internally, which significantly reduces costs.
These advantages make outsourcing the manufacturing role an attractive option, especially for companies that want to achieve fast results while minimizing risk and complexity.
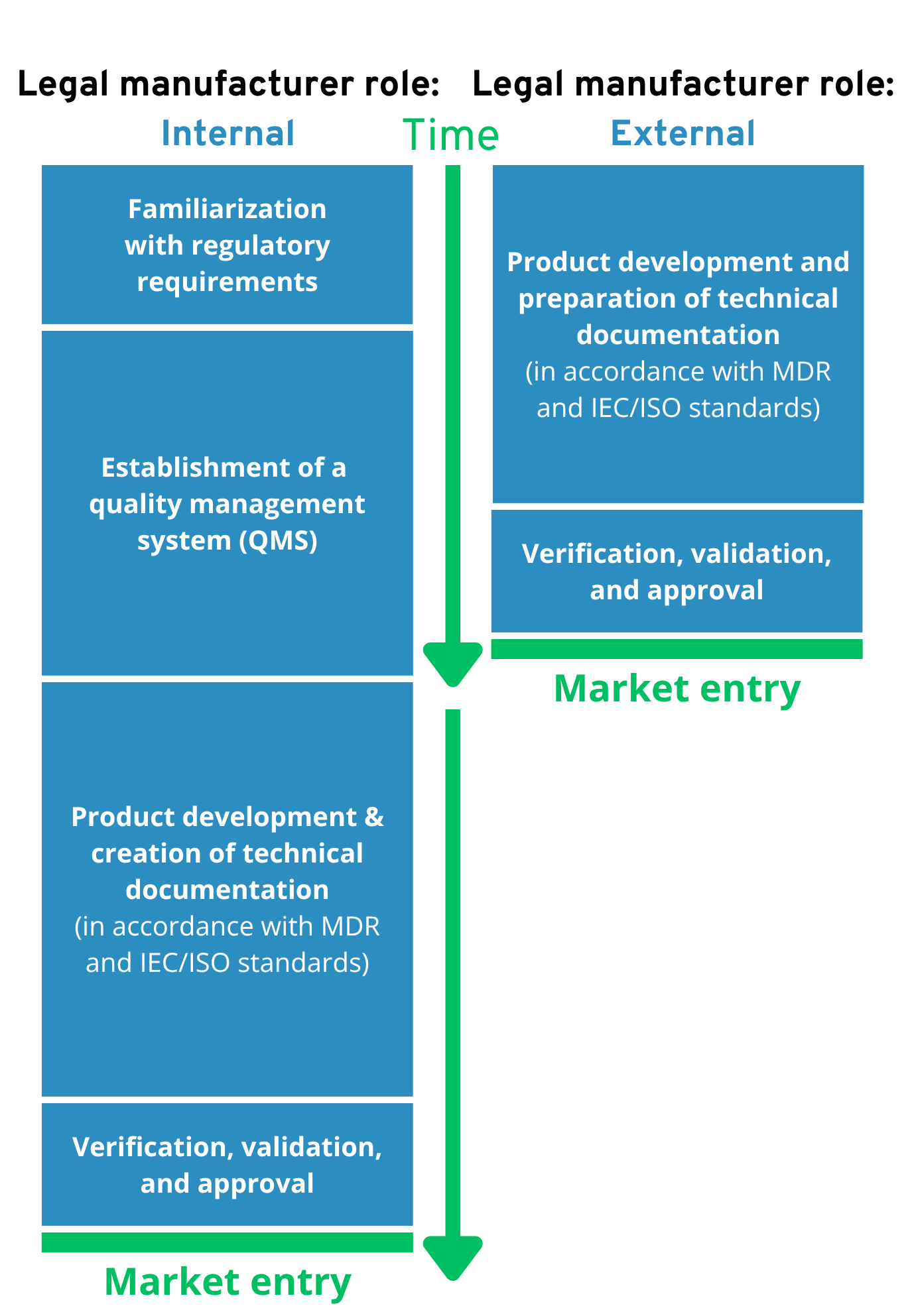
Time to market – comparison:
4. Procedure for such cooperation to outsource the role of legal manufacturer
If you would like to outsource the manufacturer role to us as a service provider, we will start by creating a customized project plan tailored to your situation. The first important step is to determine the distribution of responsibilities. To do this, you should answer the following questions, among others:
- Would you like to outsource software development or use your own internal team for this? Both options are possible in principle. We can take care of the entire product development process for you, including technical and regulatory aspects, or we can simply supplement individual services that you are unable to provide internally.
- Would you like to handle content development for the software (medical texts, images, videos, audio) yourself or outsource it? Both options are possible in principle. It is also possible for you to prepare the content and for us to take care of the final editing and visual design.
- Would you like to offer customer support (contact hotline) yourself or outsource it to us as a service provider? Both options are possible in principle. If you decide to handle this service in-house, we will train you on the relevant regulatory obligations.
Once responsibilities have been assigned, the final project plan consists of the following steps, for example:
- 1. Definition of the intended purpose and risk class according to MDR
- 2. Product development:
- a. Preparation of technical documentation in compliance with all norms and standards
- b. Software development in compliance with all norms and standards
- 3. Verification & validation of the product (including clinical assessment, summative usability evaluation, cyber security validation, etc.)
- 4. Registration of the medical device through self-certification (risk class I) or conformity assessment procedure with a notified body (risk class IIa and higher)
- 5. Further development of the product and post-market surveillance (post-market clinical follow-up, vigilance, maintenance, operation, etc.)
Please note: This process has been greatly simplified and is only intended to give you a basic understanding.
We are happy to take the lead in all of the steps mentioned above and guide you through the process. Of course, you will retain full control and make all relevant business decisions.
Get in touch:
Would you like to outsource the role of legal manufacturer?
We offer this service for software medical devices and DiGA.
Please contact us for more information: More information on how to contact us
5. Important questions and answers
Here we address important questions that experience has shown to arise when outsourcing the manufacturer role.
5.1. I would like to outsource the manufacturing role in order to bring the product to market more quickly. However, I would like to take over the manufacturing role for the product again at a later date. Is this possible?
Yes, that is absolutely possible. You retain the intellectual property (IP) rights to the product and all documentation. This means that you can re-register the product at any time and take on the role of manufacturer yourself. You benefit from a quick market launch for your product and can take over regulatory compliance in-house again in the medium term. We will help you to make this transition as smooth as possible.
5.2. Do I retain all ownership rights to the product?
Yes, you retain complete intellectual property (IP) rights to the product and documentation. QuickBird Medical simply offers you a service to help you launch a regulated software medical device. You retain full control and ownership rights to the product.
5.3. Is that more expensive than handling the topics in-house?
That depends on a variety of factors, of course. However, in our experience, outsourcing the manufacturing role leads to overall cost savings.
Cost savings can be achieved for the following reasons:
- No need to set up a QMS: The quality management system (QMS) is already in place and certified to ISO 13485 at QuickBird Medical. You don’t have to pay any costs for setting up and certifying a QMS.
- No need to set up an ISMS: The information security management system (ISMS) is already in place and certified to ISO 27001 at QuickBird Medical. You don’t have to pay any costs for setting up and certifying an ISMS.
- Trained, efficient team: The QuickBird Medical team is highly efficient in creating technical documentation for software medical devices thanks to its extensive experience in numerous projects. This means that less effort is required than if an untrained team were to take on this task.
- Peaks and troughs in capacity utilization: Experience shows that once the product has been released, the workload for the regulatory and software teams is lower than for the initial launch. The external team only incurs costs when work is actually done. An internal full-time employee must be paid continuously, even if the workload decreases.
5.4. Who is liable for the product in a regulatory sense, e.g., in the event of patient injury?
As part of the regulatory obligations under MDR, the external distributor (e.g., QuickBird Medical) assumes full liability for the product. This means that QuickBird Medical not only ensures compliance with all regulatory requirements, but also bears responsibility in the event of damage, such as injury to patients. In this way, you can successfully outsource the risk associated with medical devices.
As a medical device manufacturer, QuickBird Medical has legally binding liability insurance for personal injury. In addition, we are audited four times a year by external parties to ensure regulatory compliance.
5.5. Can the manufacturer role for a DiGA be outsourced?
Yes, QuickBird Medical is certified according to both ISO 13485 for quality management and ISO 27001 for information security management. This means we meet the requirements to act as a DiGA manufacturer and already provide this service for existing DiGA customers.
5.6. Can I subsequently outsource my manufacturer role for a certified medical device?
Yes, that is possible. We will review and transfer your existing technical documentation. The medical device can then be re-registered with us as the new legal manufacturer. Depending on whether a notified body is involved, further formalities may need to be observed.
5.7. Can I further develop the product after it has been placed on the market?
You can, of course, make changes to the product at any time. These enhancements are evaluated and then go through our quality management processes before release to ensure regulatory compliance. The whole process works in an agile development cycle for regular release of new features.
5.8. Can I continue to use my own development team?
QuickBird Medical can take over the entire development process for you and act as your external legal manufacturer in accordance with MDR.
On the other hand, it is also possible to use an internal development team at your company to implement the product and continue to outsource the manufacturer role.
6. Examples of medical device manufacturers with external distributors
To make this a little clearer, here are two examples of companies for which QuickBird Medical acts as the legal manufacturer:
- Mamly: Mamly is an approved medical device app for treating peripartum depression. It helps pregnant women reduce psychological stress through mindfulness training and professional support.
To the case study - Kontina: Kontina is an approved medical device app for treating overactive bladder (OAB) through training, educational content, and a bladder diary to reduce symptoms.
To the case study
7. Conclusion: Who benefits from outsourcing manufacturing?
When is it not advisable to outsource the role of legal manufacturer?
- You already have a lot of regulatory expertise in-house and a quality management team that is familiar with software medical devices? -> Then you should take advantage of this team and become the legal manufacturer yourself.
- You have several software medical devices on the market -> The more software medical devices you have, the greater the synergy effects of having your own quality management team.
When is it worthwhile? If you have the following goals, outsourcing the role of legal manufacturer to us as a service provider can be worthwhile:
- You want to bring your product to market as quickly as possible in order to generate sales.
- You want to avoid regulatory complexity and internal efforts that distract you from your core business.
- You want to outsource legal liability risks for the medical device.
It is important that you think strategically about which scenario makes more sense for you.
We would be happy to advise you. If you are planning medical software and are looking for a partner to implement it, please contact us at any time. As a specialized service provider, our focus is on the regulatory-compliant development of medical apps and healthcare software.
Get in touch:
Would you like to outsource the role of legal manufacturer?
We offer this service for software medical devices and DiGA.
Please contact us for more information: More information on how to contact us




