In Belgium, a special approval process for digital health applications has been established alongside Germany and France. In Austria, too, efforts to integrate DiGA into regular health care have been growing stronger since 2020. With a view to the two pioneering countries, the great potential for patient-centered medical care was identified and a special approval pathway was therefore created: the mHealth pyramid. This is intended to give the Belgian population access to quality-tested and effective digital health applications.
In this article, we take a detailed look at the steps of the approval process and you will learn which requirements need to be observed in order to successfully introduce reimbursable digital health applications in Belgium.
In addition, this article also looks at the differences and similarities between the German DiGA-Fast-Track and the Belgian mHealth pyramid: By comparing the two approaches, you will get a comprehensive overview of the different ways the two countries have taken to promote digital health applications.
A particular focus will be on the possibility of provisional approval with analogy to the German DiGA-Fast-Track.
Table of contents
- 1. Approval within the mHealth pyramid
- 2. The Belgian approval process for level 3 of the mHealth pyramid
- 2.1 Criteria for admission to level 3
- 2.2 Application procedure with the NIHDI/ INAMI
- 2.3 Procedure of the assessment for provisional approval
- 2.4 Evaluation procedure for permanent approval
- 2.5 Key aspects for non-Belgian applicants
- 2.6 Who can submit an application?
- 2.7 Which digital health applications are currently reimbursed by the NIHDI?
- 3. Overview: mHealth Level 3 versus DiGA-Fast-Track
- 4. DiGA models in other countries
- 5. Conclusion
1. Approval within the mHealth pyramid
First, we take a look at the mHealth pyramid to get a rough overview of the Belgian concept in terms of structure, responsibilities and requirements.
Since 2021, it has been mapping the approval process for a wide range of digital applications, from video consultations to digital health applications. The official title of these digital solutions is “Applications de santé mobiles” or “Applications mobiles médicales”. These are not exclusively apps for smartphone use. mHealthBELGIUM also includes pure desktop applications for medical professionals in its list of software solutions.
1.1 Current status of the Belgian approval process
The mHealth pyramid and the associated legal framework for the financing of medical apps were developed in 2016. Both were completed in January 2021 and are available as a royal decree. As this is still a new procedure in the Belgian healthcare system, adjustments are expected in the future.
A list of the digital health applications can be found on the mHealthBELGIUM website. However, the directory is only updated at irregular intervals.
1.2 Structure of the mHealth pyramid
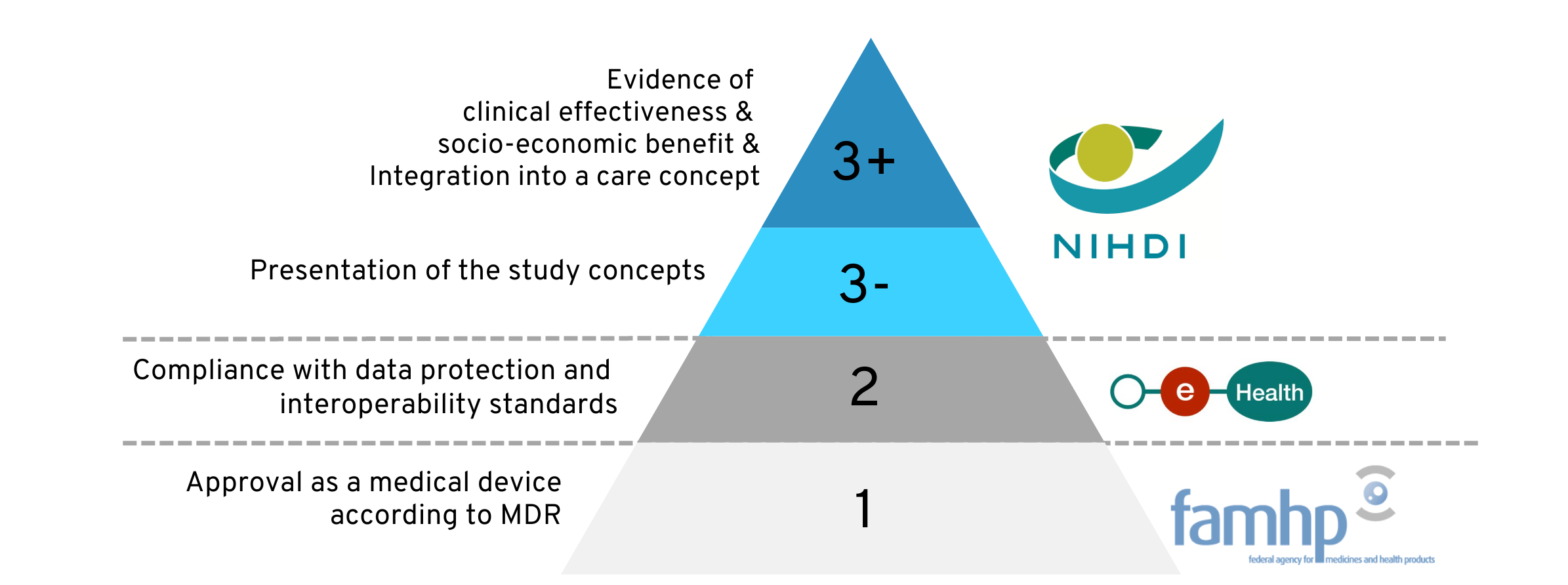
The mHealth pyramid in Belgium is structured in three stages with increasingly stringent requirements and requires interaction with various public bodies. This tier system was designed for a wide range of digital health solutions.
The illustration of the pyramid below is intended to provide an initial orientation for the following explanation. On the left-hand side, the requirements that must be met at each level are shown in broad outline. The institutions involved are listed on the right-hand side.
Of particular interest in the rest of the article are the “3-” and “3+” levels highlighted in blue: If a digital health application meets the requirements of these levels, it will be reimbursed by the Belgian health insurances.
 Approval as part of the mHealth pyramid in Belgium
Approval as part of the mHealth pyramid in Belgium
1.3 The level system of the mHealth pyramid
Once a digital application bears a CE mark as a medical device, it is mandatory for it to be approved at one of the levels of the mHealth pyramid in order to be placed on the market in Belgium. The basic clinical efficacy and safety are therefore already assured at this point.
The characteristics and purpose of your product as well as your objective with the approval will influence the level you choose:
- For digital health applications that only collect health data and transmit it to medical professionals, level 1 approval is sufficient.
- As soon as the application has a function for active monitoring of health conditions or diagnostic functions, the application must fulfill the criteria of level 2.
- Level 3 approval is then always voluntary and is only sought by the applicant if the health application is to be reimbursed by the Belgian health insurances .
As the levels build on each other, they are passed through one after the other if necessary: The certificates from one level are required for admission to the next level.
You can find more information on the individual levels in the following table:
| Admission requirement | Responsible body |
Remuneration option
|
|
| Level 1 |
|
FAHMP (Federal Agency for Drugs and Health Products) |
individual agreements with clinics or health insurance companies |
| Level 2 |
|
FAHMP (Federal Agency for Drugs and Health Products) Coordination via the so-called eHealth platform in which various institutions participate |
individual agreements with clinics or health insurance companies |
| Level 3 |
|
NIHDI (National Institute for Health and Disability Insurance) |
Reimbursement by the health insurance companies (temporary or permanent)
|
2. The Belgian approval process for level 3 of the mHealth pyramid
In the following, we focus on the requirements for the approval of a digital health application at level 3. Both the analogies and the differences to the German DiGA fast-track procedure become clear here. Below you will also find a tabular comparison between the German and Belgian process for the approval of digital health applications.
2.1 Criteria for admission to level 3
The NIHDI is responsible for the application process. Its full name is the National Institute for Health and Disability Insurance and it has a variety of tasks in the Belgian healthcare system. It also trades under the French abbreviation INAMI.
The following points are decisive for the financing of digital health applications by the Belgian health insurance companies:
- Fulfillment of the criteria from level 1 and 2
- Integration into a care concept: A key element in demonstrating added health economic value
- Clinical efficacy
- Health economic added value: The app leads, for example, to improvements in patient care, cost savings or other benefits.
For provisional admission at level “3-“, the evidence from points 2. to 4. does not yet have to be provided. However, verification concepts must be submitted with the application documents. This can be a study design, for example.
2.2 Application procedure with the NIHDI/ INAMI
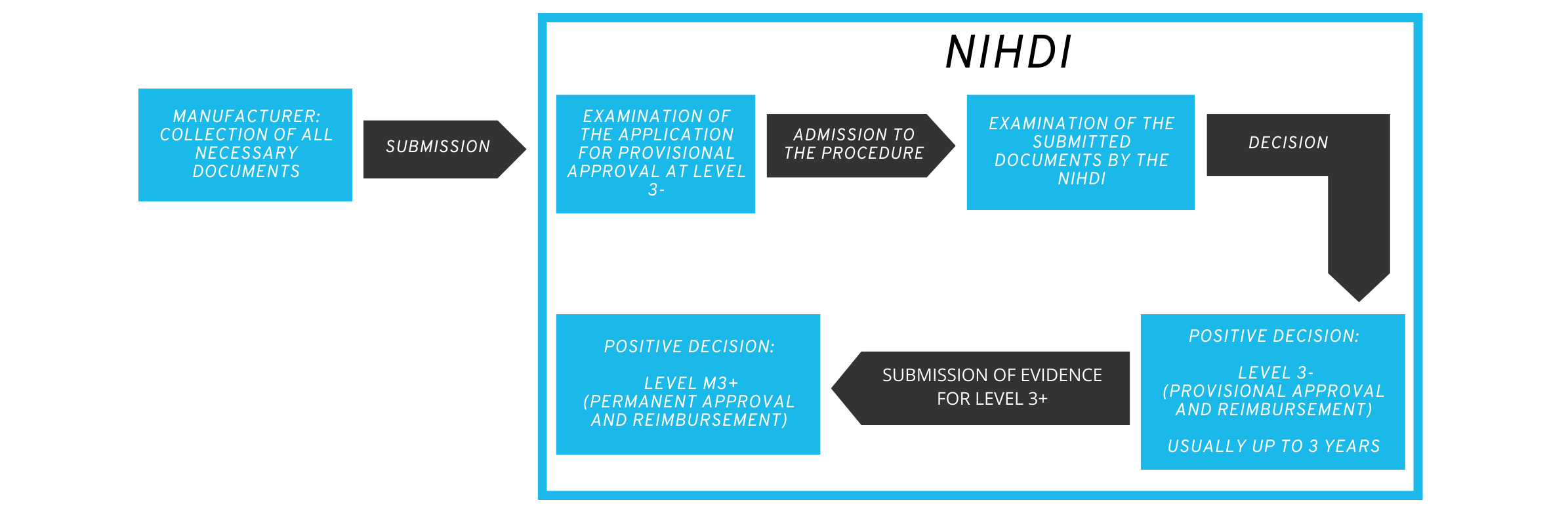
Applicants first decide whether they wish to apply for provisional or permanent reimbursement from the NIHDI and compile the dossier with the necessary documents accordingly.
Similar to the German BfArM, a multidisciplinary committee meets to evaluate the application. The committee currently comprises 13 people from various interest groups such as health insurance companies, patient representatives and members of the NIHDI.
2.3 Procedure of the assessment for provisional approval
The committee evaluates the received application for completeness and discusses the possibility of provisional reimbursement, usually in three meetings.
There are options for rejection at all stages of the procedure:
- if the application documents are not complete.
- if the provisional refund is rejected.
- if the evidence submitted is considered insufficient for a permanent refund.
During the committee’s decision-making process, manufacturers have the opportunity to explain the application dossier in person and respond to questions.
Belgium – The path from provisional to permanent reimbursement by the NIHDI
The duration of the provisional authorization is usually three years. During this time, the health application is already reimbursed by the health insurance companies. The price is negotiated by the applicant with the NIHDI.
For comparison: In Germany, the standard provisional reimbursement period is only 1 year. In addition, the price is set by the manufacturer during the provisional approval in Germany. Only when the DiGA is to be reimbursed permanently a price negotiation will take place between the manufacturer and the National Association of Statutory Health Insurance Funds.
During the provisional reimbursement phase, the applicant must report to the NIHDI after 18 months on the progress of the implementation plan for final approval. The complete documentation for the decision on permanent approval and reimbursement must be submitted no later than 6 months before the end of the trial period.
2.4 Evaluation procedure for permanent approval
If applicants decide to apply directly for permanent approval, all documents are checked for completeness by an NIHDI working group from the outset and the applicant is issued with a decision at the end.
This means that all evidence of
- clinical efficacy,
- the positive socio-economic effect,
- and successful integration into a care process
must be available in full before the application is submitted.
At the time of publication of this blog article, no direct application for permanent approval has yet been submitted in Belgium. Practical examples are therefore still missing.
2.5 Key aspects for non-Belgian applicants
A sound understanding of the Belgian healthcare system is an advantage for the successful approval of a digital health application at the NIHDI, as there are a number of special features that need to be taken into account. In comparison with the German DiGA-Fast-Track procedure, these differences stand out, which explicitly refer to the embedding of the argumentation in the context of the Belgian healthcare system:
- Integration into a care pathway
In contrast to German DiGA, digital health applications can only ever be approved and reimbursed if they are integrated into an innovative care pathway. This criterion has no exact equivalent in the German DiGA-Fast-Track.
This integration is not possible without partnerships in the Belgian healthcare system. Manufacturers can work with healthcare facilities, such as hospitals and doctors’ offices, to integrate the application into existing care processes. Pilot studies are one way of demonstrating successful integration. The benefits of the treatment can be documented via user feedback or testimonials from medical professionals. If necessary, aspects of a clinical study can also demonstrate successful integration into the Belgian healthcare landscape.
Good contacts with actors in the Belgian healthcare system are important for fulfilling this additional criterion.
- Clinical efficacy
For the marketing authorization application in Belgium, proof of clinical efficacy from a study from abroad, such as from Germany for a DiGA, can be used. The product has already provided evidence of safety and efficacy through CE marking as a medical device. However, plausible reasons must be given as to why this evidence is also valid for the Belgian healthcare system. A reference to the Belgian population and proof of the transferability of the study results are essential.
- Health economic effect:
Similar conditions also apply when demonstrating the health economic effect. While the focus of DiGA in Germany is strongly on improving patient care, Belgium also emphasizes the importance of process improvements in the healthcare system. One example of this could be the reduction of post-operative complications or more efficient patient care.
Arguments from existing documents for DiGA approval in Germany are only helpful if they can prove that the care concept presented is both innovative and superior to existing Belgian concepts.
2.6 Who can submit an application?
Since October 2023, not only manufacturers but also various other stakeholders in Belgium have been able to submit applications for the approval of digital health applications in cooperation with manufacturers. These include sales companies, hospitals, scientific associations and professional organizations.
For example, clinics that want to introduce innovative care concepts and use digital health applications as building blocks can also submit applications to the NIHDI.
The relevant application forms are available from the NIHDI.
So far, there are no practical examples of this. The extended application option will be evaluated by the NIHDI after one year. Adjustments are therefore to be expected in the future.
2.7 Which digital health applications are currently reimbursed by the NIHDI?
At the time of publication of this blog article, only the three applications of the manufacturer moveUP are reimbursed.
One of these is the smartphone app moveUP, which is used for rehabilitation after knee or hip operations. It suggests personalized exercises based on the information and transmits the information entered by the patients to the doctors treating them.
The example of moveUP clearly shows how important integration into a care pathway and the resulting socio-economic benefits are.
From the outset, moveUP was not developed in isolation, but as an integral part of a care pathway that offers benefits for both the clinic and the patient.
- Smartphone app for patients: The moveUP smartphone app provides patients with customized exercises for rehabilitation after knee or hip surgery. They also answer questions about their state of health. The data is transmitted to the attending physicians.
- Desktop application for doctors: On the doctor’s side, a desktop application is available for transferring and analyzing the data that patients record in the move Up smartphone app.
- Coaching system: The mobile app has a link to a coaching system in which patients’ questions are answered directly by medical staff. This means that the doctor’s office does not have to be visited for minor issues.
The new supply path is created by providing these three connections.
3. Overview: mHealth Level 3 versus DiGA-Fast-Track
For a better overview, the main elements of the approval routes in Belgium and Germany are compared here once again.
Similarities between level 3 of the mHealth pyramid and the German DiGA DiGA-Fast-Track procedure
| Level 3 of the mHealth pyramid |
German DiGA-Fast-Track
|
|
| Responsible authority |
NIHDI |
BfArM |
| Approval system |
Level 3 with similarities to the German DiGA-Fast-Track |
DiGA-Fast-Track process |
| Target group |
Apps with a therapeutic and/or diagnostic function |
Apps with a therapeutic and/or diagnostic function |
| Possibility of provisional approval |
yes usually for 3 years |
yes for 1 year, extension by 1 year possible in individual cases |
| Proof of positive supply effect |
mandatory for permanent approval |
mandatory for permanent approval |
| Reimbursement mechanism |
by the health insurance companies |
by the health insurance companies |
Differences between level 3 of the mHealth pyramid and the German DiGA-Fast-Track procedure
| Level 3 of the mHealth pyramid |
German DiGA-Fast-Track
|
|
| Potential applicants |
|
Manufacturer |
| Approved risk classes according to MDR |
all |
I, IIa and IIb |
| Price negotiations |
|
|
| Proof of socio-economic effect |
dictated |
no |
| Integration into existing supply paths |
dictated |
no A DiGA can be prescribed on its own. |
| Prescription required to use the app? |
partially |
yes |
| Directory of all approved apps |
out-dated directory of mHealthBELGIUM |
DiGA directory of the BfArM |
4. DiGA models in other countries
More and more countries are planning a standardized process to bring digital health applications into standard care. In addition to Belgium, you should also take a look at the following countries:
- Austria (DiGA in Austria: License for digital health applications (2024))
- France (link to the guidelines for the approval of DiGA in France)
- Germany (insider tips for the approval of DiGA in Germany)
- Italy (Link to the white paper: Will DiGA come to Italy soon?)
5. Conclusion
Belgium is very open to digitalization in many areas of the healthcare system, which is among other things reflected in the existence of the electronic patient file and the familiarization of citizens with digital care processes.
This creates an attractive market for manufacturers of digital health applications in a digitalization-friendly environment.
The Belgian authorities’ pragmatic approach to the approval of health apps can be both beneficial and challenging. On one hand, a new approval process has been created in a short period of time. On the other hand, some requirements are not yet clearly defined at the present time and further adjustments are to be expected. This dynamic situation is causing uncertainty among manufacturers.
If you are planning the approval of a digital health application in Belgium, please contact us. We help you to develop the application and find the optimal approval path.
The German DiGA fast-track program – a model for all of Europe?
In this white paper we look at which EU countries already have a DiGA model, are planning a DiGA model, or have no plans for a DiGA model. We also address whether there will be a standardized model for the EU DiGA in Europe.




