What started in Germany at the end of 2019 is now also being adopted by neighboring France: The “app on prescription,” the Digital Health Application (DiGA) is becoming an export hit – including a French equivalent to the Fast Track procedure. This provides a uniform framework for new and existing providers to be reimbursed by French health insurers for digital medical devices with direct patient benefit. We summarize for you what France requires and how to bring a DiGA to the new market.
Overview
- Background
- DMD and PECAN – at a glance
- Regulatory framework
- Actors
- Register and framework
- Procedure
- DiGA models in other countries
- Conclusion – the DMD framework as DiGA with additions
Status: July 2025
Background
Since 2019, Germany has had a uniform framework for the commonly referred to “app on prescription” in the form of the “Digital Health Application” (DiGA). In neighboring France, there are no clear regulations for the reimbursement of such applications until 2023.
Of course, it was already possible to approve software as a medical device under European regulations in France. However, there was no framework for the eligibility of digital applications for reimbursement comparable to that in Germany and no standardized process to achieve this.
France then followed Germany and a few other European countries such as Belgium in creating standardized procedures for the recognition and reimbursement of digital health applications. In Austria, too, efforts to integrate DiGA into regular health care have been growing stronger since 2020. In the future, software medical devices may be reimbursed by health insurers under certain conditions. Along the way, France is taking its cue from the German fast-track process.
DMD and PECAN – at a glance
On March 31, 2023, the French Ministry of Health published a new regulatory framework for the approval of digital health applications, that are reimbursedby french health insurance companies. In Germany, such applications are referred to as DiGA for short, while official bodies in France use the analogous term Digital Medical Device, or DMD for short.
A central part of the new regulation was the introduction of a fast-track procedure with recognizable analogies to the German “DiGA-Fast-Track”, which bears the name “PECAN”. It allows early reimbursement by health insurers for one year already without conclusive clinical evidence on the effects of the application (apart from the mandatory clinical evaluation according to MDR). At the same time, France also opened its market directly to Germany for digital medical devices of all risk classes in accordance with the MDR.
This makes France an interesting market in two respects: firstly as an export market for DiGAs already listed in Germany, and secondly as a market for digital medical devices that would not even be approved as DiGAs in Germany due to their risk class. This is because risk class IIb is the limit for DiGAs in Germany.
Regulatory framework
Basically, similar to Germany, not every medical device is a DMD, but every DMD is a medical device. Basically, similar to Germany, not every medical device is a DMD, but every DMD is a medical device.
In principle, approval as a Digital Medical Device (DMD) in France requires the presence of a European CE certification, which refers to the Medical Device Regulation (MDR). For more information, see our blog article on MDR approval & certification.
Innovative clinical or organizational benefit
The central requirement for recognition is an innovative component either in terms of clinical benefit for the patient or in terms of organizational improvements for the healthcare system.
Interoperability
Equally comparable to the German requirements is the obligation to meet the interoperability and safety standards that the French government lists here. Among other things, this places demands on the portability of health data, the identification of users and special data protection. A key component is the embedding in the “French National eHealth ID” (INS), which enables uniform identification and management of personal master and health data.
Risk classes
One of the most important differences between the German DiGA and the French DMD regulation is the opening to risk class III. Only products in risk classes I to IIb can be listed as German DiGAs, while risk class III is also included in France.
Personal data protection and data security
A DMD in France must comply with national and international data protection law. Nationally, the authoritative legal standard is Act 78-17; internationally, it is the European data protection standard DSGVO. In addition, it is mandatory that all providers hosting personal health data – i.e., in the case of a DMD, the corresponding cloud service provider – have undergone “Hébergeurs de Données de Santé (HDS)” certification; Microsoft offers this, for example.
Proof of actual usage
It also calls for using an individual activation code to prove that the prescription was actually filled, so that DMD providers receive their reimbursement from French health insurers based on actual usage.
DiGA Fast Track vs. DMD and PECAN: Differences
The following chart presents the key differences between the DiGA Fast Track and DMD with the PECAN process in France. An important difference here is that DiGA can currently only be medical devices in risk classes I, IIa and IIb according to MDR (in the future possibly also class III), while France also allows class III for DMD in the PECAN procedure.

Actors
Four main actors, hierarchically subdivided, play a role in DMD approval in France:
• The Ministry of Health and Prevention, as the highest authority, is responsible for setting the general regulatory framework. In addition, it is responsible for the final decision on the approval of a DMD.
• The Agence Nationale de Sécurité du Médicament et des produits de santé is responsible for the final approval of medical devices in France.
• The Agence du Numérique en Santé (ANS) is the executive body on behalf of the Ministry of Health specifically responsible for Digital Health. Fast track DMD testing is the responsibility of the SNE.
• The Commission nationale d’évaluation des dispositifs médicaux et des technologies de santé (CNEDiMtS) is responsible for the testing and evaluation of medical devices and applications, including digital health applications. Their assessment is the primary basis for the SNE’s review. The main pillar of their review is the technical specification of the software and an estimate of how large the number of potential users for the respective indication is most likely to be.
Register and framework
Depending on the type of DMD, it is assigned to one of two registers.
- LATM includes all digital medical devices whose main component is the monitoring of health data. This is a key difference to the German DiGA, which excludes (tele)monitoring and data collection/processing as the primary purpose of the app.
- LPPR is the list of all reimbursed medical devices for therapeutic purposes.
The division into two different registers is a key difference to the German model, which has only one central register specifically for all DiGAs.
Excursus:
Mon Espace Santé: In addition, as early as 2022, the Republic of France created “Mon Espace Santé”, a one-stop digital shop that allows all citizens to securely manage their health data digitally, and also opens a way to share it with doctors, clinics, practices – but also with listed digital services, which may include DMD providers.
This database includes medical history, current health data (such as weight, blood pressure, etc.) and important health documents.
Particularly interesting: A listing in this catalog as an eHealth provider enables, in addition to visibility for patients and the “soft factor” of gaining trust, above all the use of the health data deposited by the patient, provided that the patient has given their consent.
Procedure
The path to reimbursable DMD can be taken in two stages using the fast-track process, but theoretically the direct path to permanent approval is also conceivable.
The fast-track procedure (PECAN) is the first step towards recognition as a DMD, and is strongly based on the German fast-track model. Within only two months, the CNEDiMTS issues a statement on the basis of which the French Ministry of Health decides on “early reimbursement” and its framework conditions – this still without the existence of a study and thus proven clinical evidence (however, at least “initial clinical evidence” seems to be required here as well). The trial period cannot be extended, so full recognition under the regular procedure is required after one year at the latest. By then, therefore, there must be sufficient clinical evidence of the positive effect of the application.
In the first step of the PECAN process, the application is sent to the French Ministry of Health and, as a copy, also to CNEDiMTS. The proof of compliance is obtained by the ANS.
In the second step, the CNEDiMTS provides its assessment of whether and to what extent DMD provides health and/or organizational benefits to the health care system. It is important to note that this is indeed “only” an assessment (albeit a very weighty one). This assessment is also accompanied by the Certificate of Compliance from the ANS, provided that the ANS has determined that the requirements in terms of interoperability and safety have been met.
Based on both assessments or findings, the French Ministry of Health and Social Affairs ultimately makes a decision. If the outcome is positive, the DMD is reimbursable for one year (non-renewable) at a set price. This completes the fast track process.
Regular testing of DMD by CNEDiMTS lasts either six months (for a therapeutic use) or three months (for a monitoring use). The reimbursability established in the case of successful validation then applies permanently.
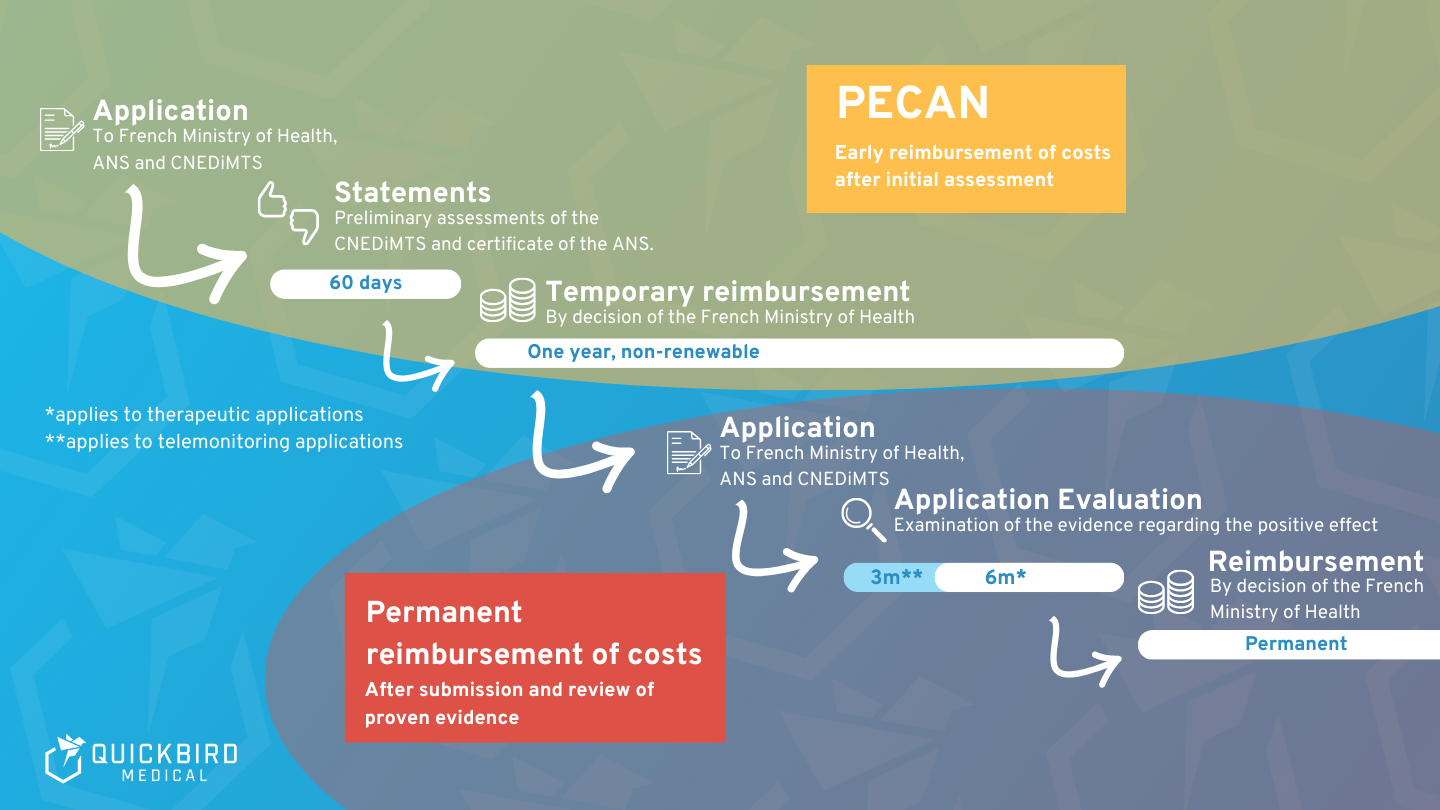
PECAN as admission on trial
On the application side, there are differences in terms of time requirements and complexity. Permanent reimbursement already requires that scientific evidence (in the form of one or more studies) on the medical and/or organizational benefit to the health care system (cf. “positive health care effect” in Germany) is already available and submitted, whereas the PECAN process replaces this requirement with the assessment of the CNEDiMTS.
The application is thus considerably simplified in the first step, and the processing times are correspondingly greatly reduced – instead of six (for a therapeutic product) or nine (for a telemonitoring product) months, the opinion of CNEDiMTS in the PECAN procedure takes merely two months.
Under the simplified and accelerated PECAN procedure, approval is granted for a limited period of one year and cannot be extended – while the subsequent approval for permanent admission is valid for several years and can be renewed.
PECAN should therefore be seen as a bridge to the actual, permanent approval. The PECAN process provides DMD startups with time, financial flexibility, and the opportunity to produce evidence during the “pilot” phase.
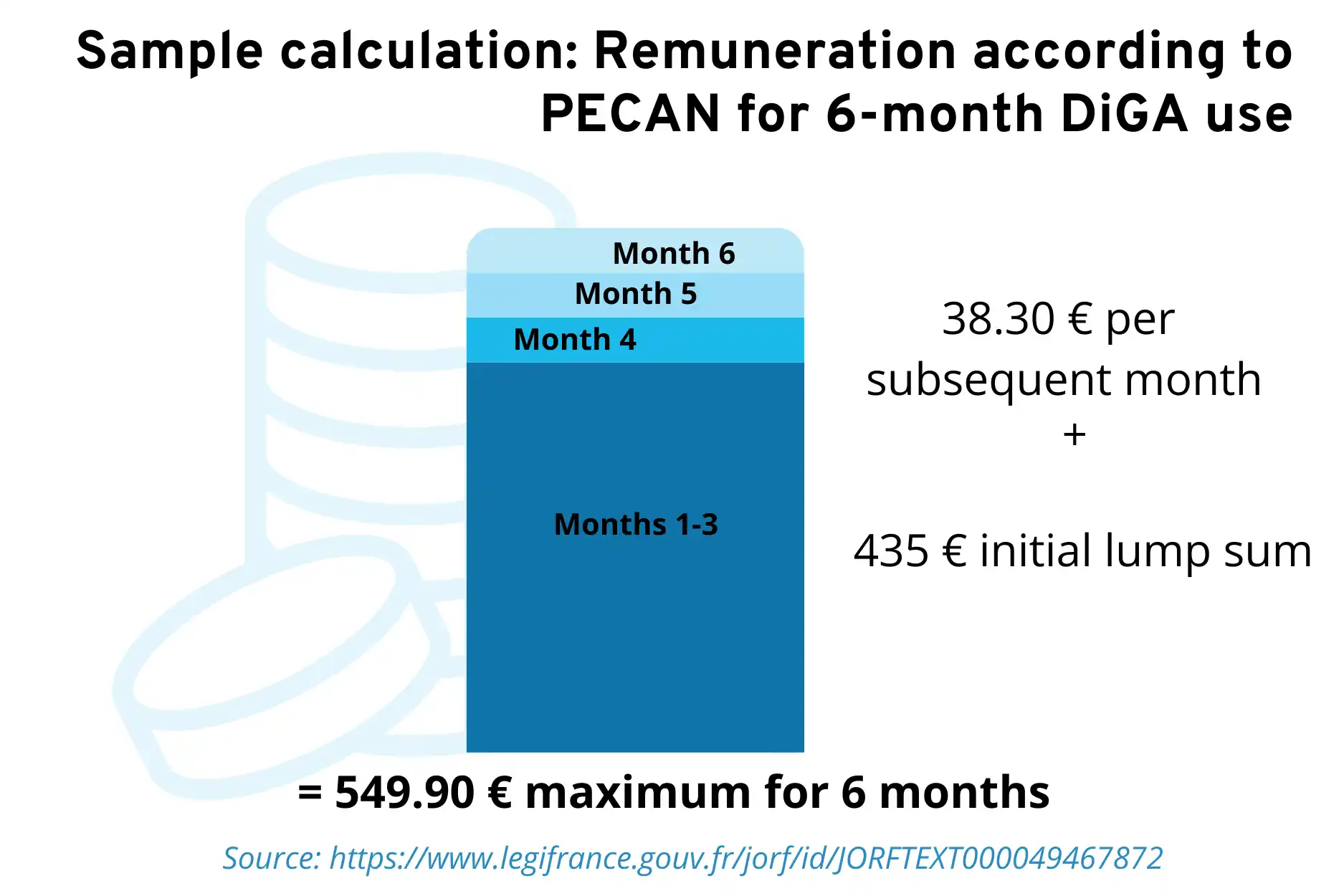
Remuneration amounts during PECAN
The provisional renumeration amounts have also been set since April 2025.
- a one-off initial flat rate of €435 (incl. VAT) for a maximum period of use of three months
- a monthyl lump sum of €38,30 (incl. VAT), which is charged after the initial three months of used
- a maximum amount of €780 (incl. VAT) per year
Sample calculation for the remuneration of PECAN:
Further plans for PECAN
In the draft for the coming Law on the financing of social security (PLFSS) the French government is planning several adjustments to the PECAN procedure as of July 2025.
The plans include:
- Extension of the refund periodfrom the current one year to two years in future.
- Extension of the scope to digital applications to compensate for disabilities (beyond digital therapeutics and telemonitoring
- Examination of a simplification of the application requirementsin the approval procedure
We will keep you up to date on further developments.
DiGA models in other countries
More and more countries are planning a standardized process to bring digital health applications into standard care. In addition to France, you should also take a look at the following countries:
- Belgium (link to the guidelines for the approval of DiGA in Belgium)
- Austria (DiGA in Austria: License for digital health applications (2024))
- Germany (insider tips for the approval of DiGA in Germany)
- Italy (Link to the white paper: Will DiGA come to Italy soon?)
- Switzerland (reimbursement of digital health applications (DiGA & dGA))
In addition to standardized DiGA approval processes, there is also the option of having digital solutions reimbursed via selective contracts. Find out more in our white paper on selective contracts.
Conclusion – the DMD framework as DiGA with additions
France is largely adopting the German DiGA concept, including Fast Track, and is thus facilitating accelerated and simplified market entry for reimbursable digital medical devices (DMD). The requirements for qualifying a medical device for DMD are largely the same as those for the German market.
However, there are still uncertainties regarding the assessment criteria (as of 2025): It is neither officially known which exact forms of study are required, nor along which guard rails the prices will be set or negotiated; the law remains unclear at this point and refers to ministerial decisions that are yet to follow.
Nevertheless, one thing is already clear: the introduction of the legal framework for the standardized handling of reimbursable DMDs in France has opened up a wide field for German or German-based providers who can bring their already established DiGAs to another market with a population of around 67 million – an interesting opportunity to diversify development costs.
In addition, the more liberal handling of risk classes offers yet another opportunity. Digital medical devices, which are currently excluded from the DiGA definition in Germany due to risk class III, are being given a chance in France. Thus, the new regulation in our neighboring country enables the establishment of products and ultimately business models that are currently unthinkable in this country under DiGA regulation.
This and the general fact that France is recognizably showing great ambition in the further development of the framework for digital health makes it seem worthwhile for DiGA providers or aspirants to look beyond the western German border.
QuickBird Medical is happy to advise and assist you in the implementation of your DMD for the French market.
The German DiGA fast-track program – a model for all of Europe?
In this white paper, we look at which EU countries already have a DiGA model, are planning a DiGA model or have no DiGA model in prospect. We also clarify the question of whether there will be a standardized model in Europe for the EU DiGA.




