Development of Decision Support Systems (2026)
We develop customized software systems for your company that support specialists in the diagnosis or treatment of diseases.
Holistic Implementation of
Decision Support Systems
Software Development
As a reliable partner, we support you from the initial idea through the development of your software to go-live and beyond. We create applications that your users can rely on.
Medical Device Certification
We have many years of experience in the development of medical software as a medical device. Our quality management system is certified according to ISO 13485 and our software life cycle is in accordance with IEC 62304.
UI / UX Design
We design applications that help doctors and HCPs make better and faster decisions. We combine in-depth knowledge of medical processes with cutting-edge design principles to develop applications that integrate harmoniously into everyday working life.
Our Process
Strategy Consulting & Feasibility Analysis
Together with you, we develop a suitable product strategy, the business model and the basic concept of the decision support software. We identify the opportunities and risks of various solutions and provide you with comprehensive advice.
Concept & Design
We create a design concept based on your ideas, all regulatory requirements and user needs. Design work is integrated into the product development cycle in an agile manner and continuously optimized.
Implementation & Testing
In an agile process, we develop a software product for you that is ready for use by HCPs. We ensure that your users can rely on the product and that data security is covered on the basis of BSI guidelines.
Maintenance & Support
Of course, we won’t let you down even after your product has been published. Our team remains at your side to maintain your software on an ongoing basis, operate your server and continuously integrate new features according to your individual requirements.
Key Aspects of CDS Development
Connection to HIS
We integrate your software into the leading hospital information systems (HIS) in the German healthcare industry.
Usability von Decision-Support-Systemen
We ensure that your software is accepted and applicable by doctors in practice.
Cyber Security
We ensure that health data is secure at all times – in accordance with ISO/IEC 27001, ISO 81001-5-1, MDCG 2019-16.
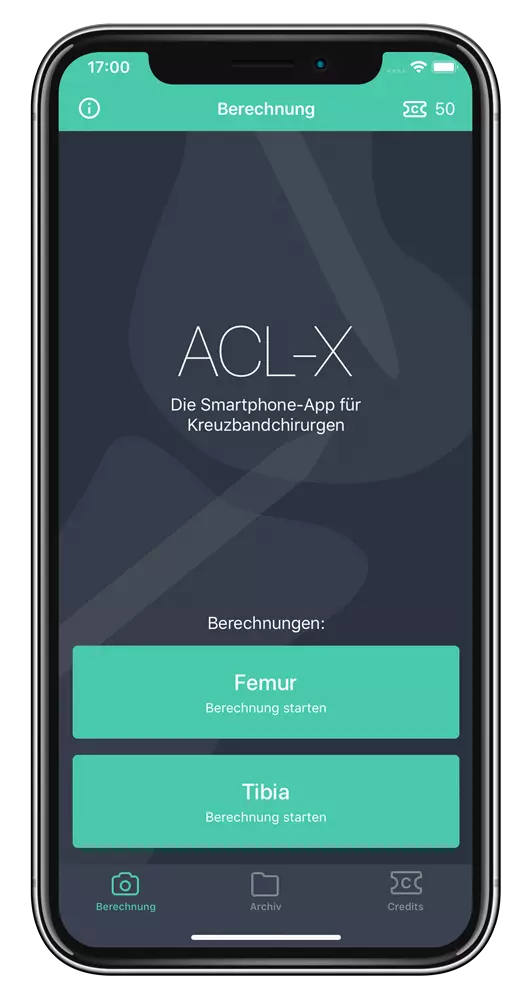
ACL-X
Digital support for knee surgery
ACL-X simplifies the intraoperative procedure for cruciate ligament injuries in the knee joint and makes it more accurate and reliable. The surgeon (or an assistant) can import the X-ray image into the ACL-X app and position virtual guide lines that can be used to precisely reference and quantify the actual drill wire position in the femur and tibia to the X-ray anatomy.
TEDIRO
Therapy Management System for Robotic Gait Training
The THERY robot from TEDIRO opens a new chapter in gait rehabilitation by enabling patients to perform autonomous gait training following surgery on the lower extremities. The Therapy Management System developed by QuickBird Medical automates the planning, scheduling, and implementation of innovative gait training in clinics.
What really sets us apart

ISO 13485 certified
Our quality management system is certified according to ISO 13485. This enables us to ensure the compliant development of software as a medical device and to meet the requirements of the MDR and FDA (21 CFR) for quality management systems.

Legal Manufacturer for your Medical Device
On request, we can take on the role of legal manufacturer for your medical device software and thus bear the legal responsibility for compliance with all regulatory requirements of the MDR.

ISO 27001 certified
QuickBird Medical is ISO 27001 certified for information security. We are experts in the areas of cybersecurity and health data protection.
Whitepaper
13 important recommendations for aspiring software medical device manufacturers in 2026
- Tips for planning and implementing your medical software or app
- Practical advice for the initial market launch
- How to implement regulatory requirements pragmatically
- What risks you can avoid
- Why risk class I is possible under MDR
Are you planning to implement
a Decision Support System?
Our team supports you in the planning, design and technical implementation of your application. Contact us and arrange a no-obligation initial meeting.