Since the DiGA concept was launched, we have learned a great deal about the development of digital health applications. We share as much of this knowledge as possible with (prospective) DiGA manufacturers through our specialist articles. This means that not everyone has to go through the same painful learning process two or three times over.
Since we develop DiGA on a contract basis for start-ups, pharmaceutical companies, and other businesses, we see the entire development cycle of a DiGA: from the initial idea to permanent approval and beyond.
In this article, we would like to give you an overview of all the topics we have already written about. You will find an overview of all guides and specialist articles, sorted by topic.
Tip: If you are new to this subject, it makes sense to simply work through the content chronologically from top to bottom.
Table of contents
- 1. DiGA: Development & Approval
- 1.1 Is your app a DiGA? Definition criteria of digital health applications
- 1.2 12 Important recommendations for prospective DiGA manufacturers
- 1.3 Guide to DiGAV: Digital Health Applications Ordinance
- 1.4 DiGA: Guidelines for the proof of positive healthcare effects
- 1.5 DiGA Guide: History of all changes
- 1.6 Interoperability for digital health applications (DiGA)
- 1.7 Data security and data protection certificates for DiGA
- 1.8 Approving DiGA & software medical devices without quality management & regulation?
- 2. DiGA: Finance, business model, marketing, and sales
- 3. DiGA: Statistics and market situation
- 4. DiGA: Models in Europe
- 5. Medical device development and approval
- 6. Alternative reimbursement options to DiGA
- 6.1 Selective contracts with health insurance companies: The alternative to DiGA
- 6.2 Certification of digital courses according to ZPP – Central Testing Agency for Prevention
- 6.3 DiPA: digital applications for nursing care
- 6.4 Reimbursement for software in the GKV list of medical aids (HMV)
- 6.5 Innovation funds and other subsidies for health software
- 7. Further current information on the development of DiGA and medical device software
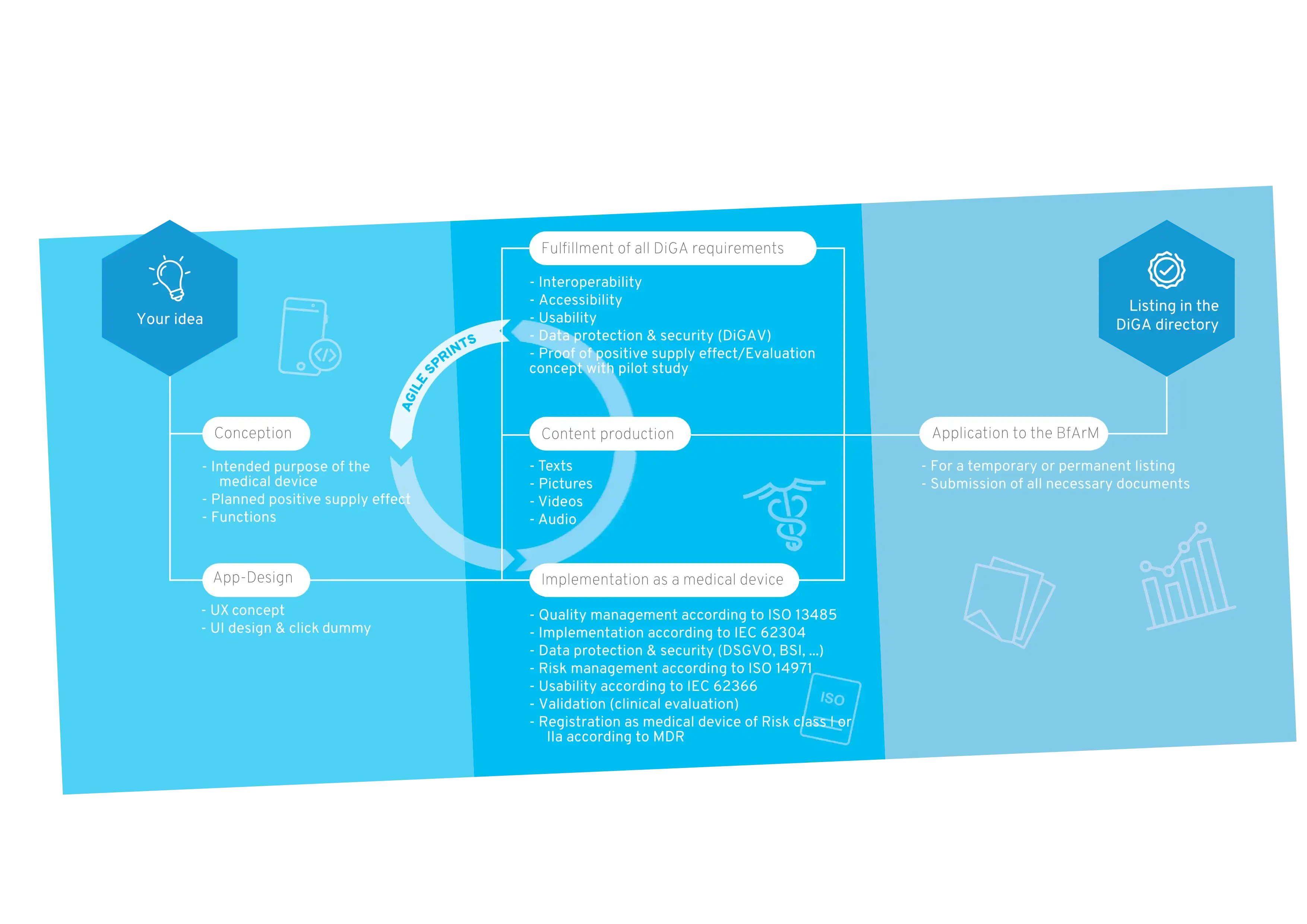
1. DiGA: Development & Approval
The path to the DiGA directory
The following technical articles and guidelines deal with various aspects of the technical and regulatory development of DiGA:
1.1 Is your app a DiGA? Definition criteria of digital health applications
At the beginning of your planning, the first question that arises is whether your application is actually a DiGA. In other words, does it meet all the criteria to qualify as a DiGA?
1.2 12 Important recommendations for prospective DiGA manufacturers
Before you start developing a DiGA, you should learn from the mistakes of other DiGA manufacturers to avoid them in your own project. We provide exclusive insights and important recommendations from practice that you should consider in your planning:
1.3 Guide to DiGAV: Digital Health Applications Ordinance
The DiGAV forms the basis for the legal requirements for digital health applications. Here you will find an overview of what these requirements are.
1.4 DiGA: Guidelines for the proof of positive healthcare effects
In this article, we provide detailed guidelines on how you can prove positive healthcare effects. This includes a pilot study, evaluation concept, and comprehensive study for the permanent inclusion of DiGA.
1.5 DiGA Guide: History of all changes
The official DiGA guideline is probably the most important document that every DiGA manufacturer should read. The BfArM also updates this guideline regularly. Unfortunately, there is no detailed change history available for this yet. Therefore, we continuously review all changes to the DiGA guideline and present all changes with images and comments in a clear manner for you here.
1.6 Interoperability for digital health applications (DiGA)
A DiGA is subject to a number of legal interoperability requirements. In this article, you will learn more about these requirements and gain practical insights into the implementation of interoperability standards for a DiGA.
1.7 Data security and data protection certificates for DiGA
Digital health applications must meet strict data protection and data security standards to ensure the integrity and confidentiality of patient-related data. Our article provides a comprehensive overview of the necessary certificates and how they can be obtained.
1.8 Approving DiGA & software medical devices without quality management & regulation?
DiGA and the Medical Device Regulation (MDR) present manufacturers with comprehensive challenges. These include setting up a quality management system (QMS), creating detailed technical documentation, and managing increased regulatory liability risks. In this context, many companies are considering whether they can delegate the extensive regulatory requirements for medical devices entirely to an external provider.
This is indeed entirely possible by outsourcing the role of legal manufacturer to an external company.
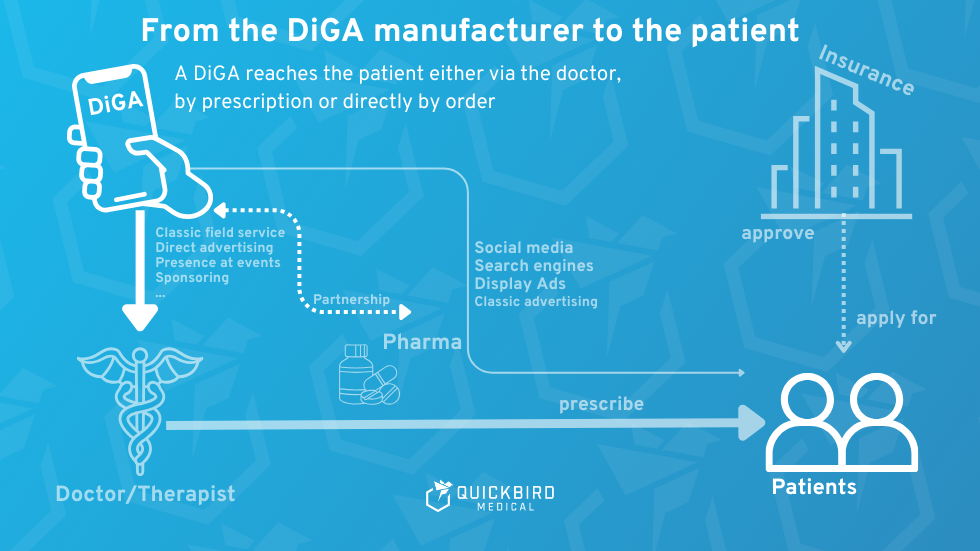
2. DiGA: Finance, business model, marketing, and sales
Graphic of the possible DiGA distribution channels
2.1 DiGA price negotiations with the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds)
This article describes how price negotiations for DiGA are conducted with the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds). It explains the differences between the provisional and permanent inclusion of a DiGA in the directory and the associated time frames for the start of negotiations. The article provides an overview of the necessary documents and the duration of the negotiations, as well as the role of the arbitration board in the event that no agreement is reached.
2.2 How you can use DiGA data for the distribution and R&D of medicinal products
Pharmaceutical companies benefit greatly from real-world data about the target groups for their drugs. Such data is particularly valuable for the distribution of existing drugs and the development of new products.
But how can you use a DiGA to collect data that can then be used, for example, for research into the provision of drugs?
2.3 DiGA revenue & costs: Is DiGA worth it?
When launching a DiGA, it is important to assess its financial potential. The key question is: Does developing your DiGA idea make financial sense? This is also what potential investors will be asking themselves. You should therefore present convincing calculations that include profit, revenue potential, market potential, and costs. This article provides detailed instructions and sample calculations to help you accurately determine the potential of your DiGA for the selected disease indication.
2.4 Guide: DiGA marketing and sales
Even if all regulatory requirements are met and your DiGA is listed in the central directory, this does not guarantee successful market entry. Simply being listed does not automatically mean that doctors will prescribe your DiGA. That is why choosing effective marketing and sales strategies is crucial to exploiting the full market potential of your niche. The range of possibilities is wide and the market environment complex. This article provides an overview and practical tips.
3. DiGA: Statistics and market situation
3.1 The DiGA directory for manufacturers
Digital health applications (DiGA) can be compared based on various criteria such as the medical device risk class, the responsible regulatory authority, the duration of the DiGA prescription, or the list price. Unlike the BfArM directory, our DiGA Analyzer offers search filters specially adapted for manufacturers, enabling targeted market analysis.
→ Link to DiGA directory analysis
DiGA statistics: Currently listed DiGA
DiGA statistics: The DiGA directory in figures
Analyze the DiGA directory using our real-time dashboard. Discover up-to-date information on risk class distribution, prices and costs, prescription duration, and other important statistics about the DiGA landscape.
3.2 Current DiGA Reports 2025: Overview of official reports
The latest figures and reports on approved digital health applications (DiGA) are extremely important for strategic planning and market analysis of new DiGA projects.
Prescription figures and other statistics can be found in reports from various institutions such as the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds) or the Spitzenverband Digitale Gesundheitsversorgung (National Association for Digital Health Care).
Since the information may be scattered, this article provides a chronological overview and links directly to the DiGA reports of the most important publishers.
4. DiGA: Models in Europe
Germany broke new ground in 2019 by introducing a standardized model for the approval of digital health applications (DiGA). Other countries in Europe are now following suit and are also developing DiGA models or have already implemented them.
In the following articles and white papers, we take a look at which countries …
already have a DiGA model in place,
are planning a DiGA model,
or have no DiGA model in the pipeline.
→ DiGA & DTX in Italy
→ DiGA & DTX in Austria: Approval of digital health applications (2025)
→ DiGA & DT in Switzerland
→ DiGA & DTX in France
→ DiGA & DTX in Belgium
→ DiGA & DTX in the EU: The DiGA fast track – soon to be available across Europe?
5. Medical device development and approval
Before being approved as a DiGA, your product must be approved as a medical device in accordance with the Medical Device Regulation (MDR). In addition to the requirements of the DiGA Regulation, this brings with it many challenges within the framework of the MDR.
We have written a large number of specialist articles dealing specifically with the approval of software medical devices.
→ Link to overview of all articles on medical device development and approval
6. Alternative reimbursement options to DiGA
Our white paper ‘All paths to reimbursement for medical software and health apps (in 2025)’ provides an overview of all paths to reimbursement.
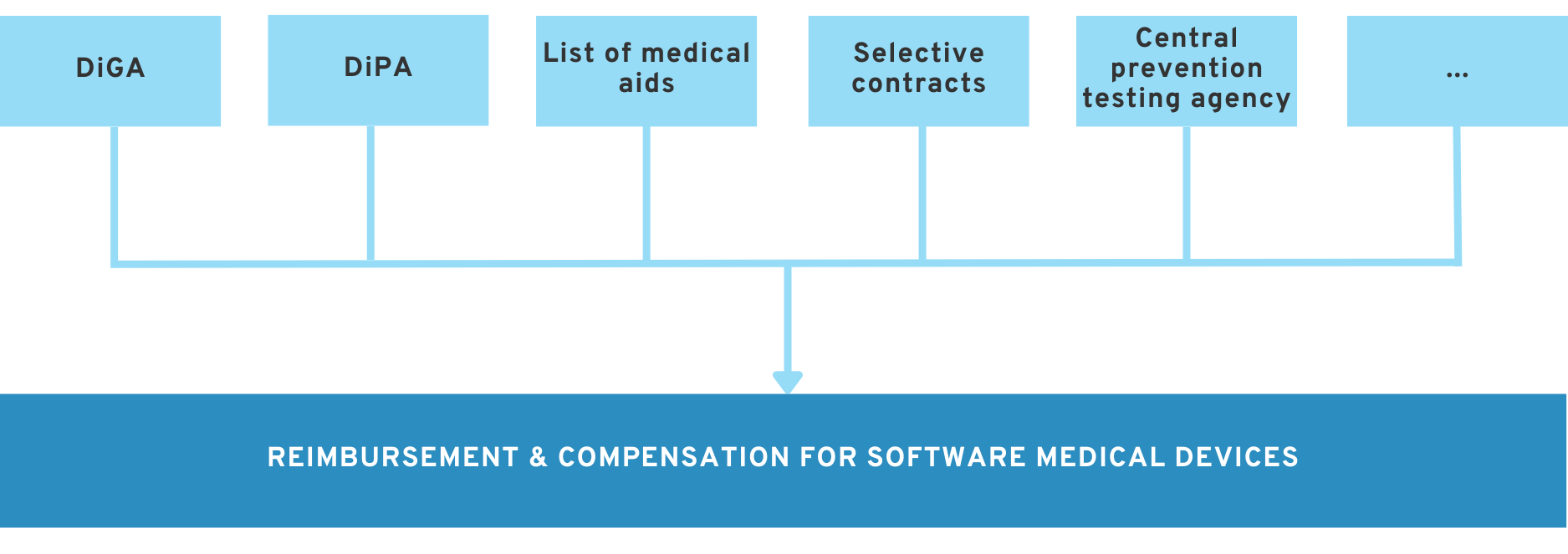
In addition to DiGA approval, there are a number of other ways to get DTx or digital solutions reimbursed by health insurance companies. The DiGA approval process is not the best option for every manufacturer.
In the following articles, we will discuss other reimbursement options in the German healthcare system.
6.1 Selective contracts with health insurance companies: The alternative to DiGA
Selective agreements are an exciting alternative to the highly regulated DiGA approval process.
6.2 Certification of digital courses according to ZPP – Central Testing Agency for Prevention
Manufacturers who develop products for disease prevention should consider certification by the Central Testing Agency for Prevention.
6.3 DiPA: digital applications for nursing care
DiPA is the equivalent of DiGA in the care sector. Find out how you can get DiPA reimbursed.
6.4 Reimbursement for software in the GKV list of medical aids (HMV)
Software products that are considered medical aids and compensate for physical impairments, for example, could be included in the list of medical aids. This opens up another avenue for reimbursement by health insurance companies.
6.5 Innovation funds and other subsidies for health software
There are various public funding programs available for legal manufacturers of digital health solutions, depending on the project focus, maturity, and target market. One of the most important and practical sources of funding in the German healthcare system is undoubtedly the G-BA Innovation Fund, which specifically supports new forms of care and offers attractive opportunities for software projects related to healthcare.
7. Further current information on the development of DiGA and medical device software
A lot of work and time has gone into the guides and articles. We hope that they will be of some help to you as a manufacturer in meeting your challenges.
Finally, we would like to recommend our newsletter, in which we send out a detailed report on current topics in the field of DiGA and medical device development every month: To the newsletter
You can also find the latest developments and news on DiGA & medical software on our news page.