The Digital Care Act for “better care through digitization and innovation” came into force on December 19, 2019. The aim of the Digital Care Act (DVG) is to enable patients to have health applications prescribed by their doctor, make telemedicine more accessible, and promote digital innovation. The DVG also initiated the introduction of electronic patient records (on the telematics infrastructure) in January 2021.
In this article, we explain what is meant by “doctors can prescribe apps” and, specifically, how software manufacturers can have their apps or software prescribed. The DVG not only makes financing apps easier, but is also intended to significantly speed up the negotiation process with health insurance companies.
Table of contents
- 1. Prescribing apps
- 2. Accelerated market launch
- 3. Criteria for inclusion in the directory of digital health applications
- 4. Conclusion
1. Prescribing apps
Basically, we are not talking about apps, but about health applications in general. These are medical devices that use digital technologies to support the detection, monitoring, treatment, compensation, or alleviation of diseases, injuries, or disabilities. These include, for example, apps that help diabetes patients document their blood sugar levels or apps that remind their patients to take their medication.
Patients will be able to obtain such applications in the future through a prescription from their treating physician or with the approval of their health insurance company. The app manufacturer will receive appropriate financial compensation for this.
To do so, however, the app manufacturer must first submit its app to the BfArM (Federal Institute for Drugs and Medical Devices) for review. If the BfArM approves, the app will be covered by statutory health insurance for a provisional period of one year. Once a positive effect on care has been demonstrated, the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds) will negotiate with the manufacturer to determine the amount of reimbursement by the health insurance funds. Prior to permanent inclusion, the prices set by the manufacturer apply.
2. Accelerated market launch
Another important point is the accelerated market launch: The BfArM decides on the application within three months of receiving the complete application documents. If the BfArM accepts the application/app, it is added to the DiGA directory (digital health applications).
Once the app has been permanently approved, negotiations with the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds) begin to determine the reimbursement amount. Here, too, there is a clear timeline: if no agreement is reached within six months of the start of negotiations, the arbitration board will determine the reimbursement amounts within three months.
If the health application meets all criteria, the way is clear for a rapid market launch. This makes it easier for startups in particular to enter the already difficult healthcare market.
3. Criteria for inclusion in the directory of digital health applications
Akzeptiert das BfArM die Anwendung/App, wird sie in das DiGA-Verzeichnis (Digitale Gesundheits-Anwendungen) aufgenommen. Für die Aufnahme muss die Anwendung laut Aussage des BfArM u.a. folgende Kriterien erfüllen. Alle Details zum Verfahren inkl. der genauen Kriterien zur Aufnahme in das DiGA-Verzeichnis werden in einer ergänzenden Rechtsverordnung behandelt. Diese Rechtsverordnung nennt sich DiGAV (Digitale-Gesundheitsanwendungen-Verordnung). Wir haben zur DiGAV hier einen umfangreichen Leitfaden geschrieben. Hier ist schon mal ein grober Überblick:
#1: The application must be a low-risk medical device (Class I or IIa) and have successfully completed a conformity assessment procedure in accordance with MDR (Medical Device Regulation) or MDD (Medical Device Directive).
This criterion is very clear and compliance is specifically regulated by the MDR and MDD. We explain how you can decide whether your application is a medical device in a separate article. If you already know that it is a medical device, you can find its risk class using our MDR guide.
#2: Positive effects on care must be demonstrated through application.
The DiGA must demonstrate positive effects on healthcare. If the manufacturer is initially unable to demonstrate any positive effects on healthcare, it can still apply for the digital health application to be included in the DiGA directory for testing. With sufficient justification, this period can be extended by up to 12 additional months. More information on this can be found in our guide to the DiGAV.
#3: The application is tested for security, functionality, and quality.
Since the application must be a medical device, this criterion will naturally be met. There is currently no better proof of safety, functionality, and quality for health applications than the Medical Devices Act/Medical Devices Regulation.
#4: The application must ensure data security and data protection.
A fundamental distinction is made between data security and data protection. Data security refers to technical safeguards for protecting data (against viruses, manipulation, hackers, etc.). Data protection describes how personal data can be processed in order to protect it from misuse (regulated by law).
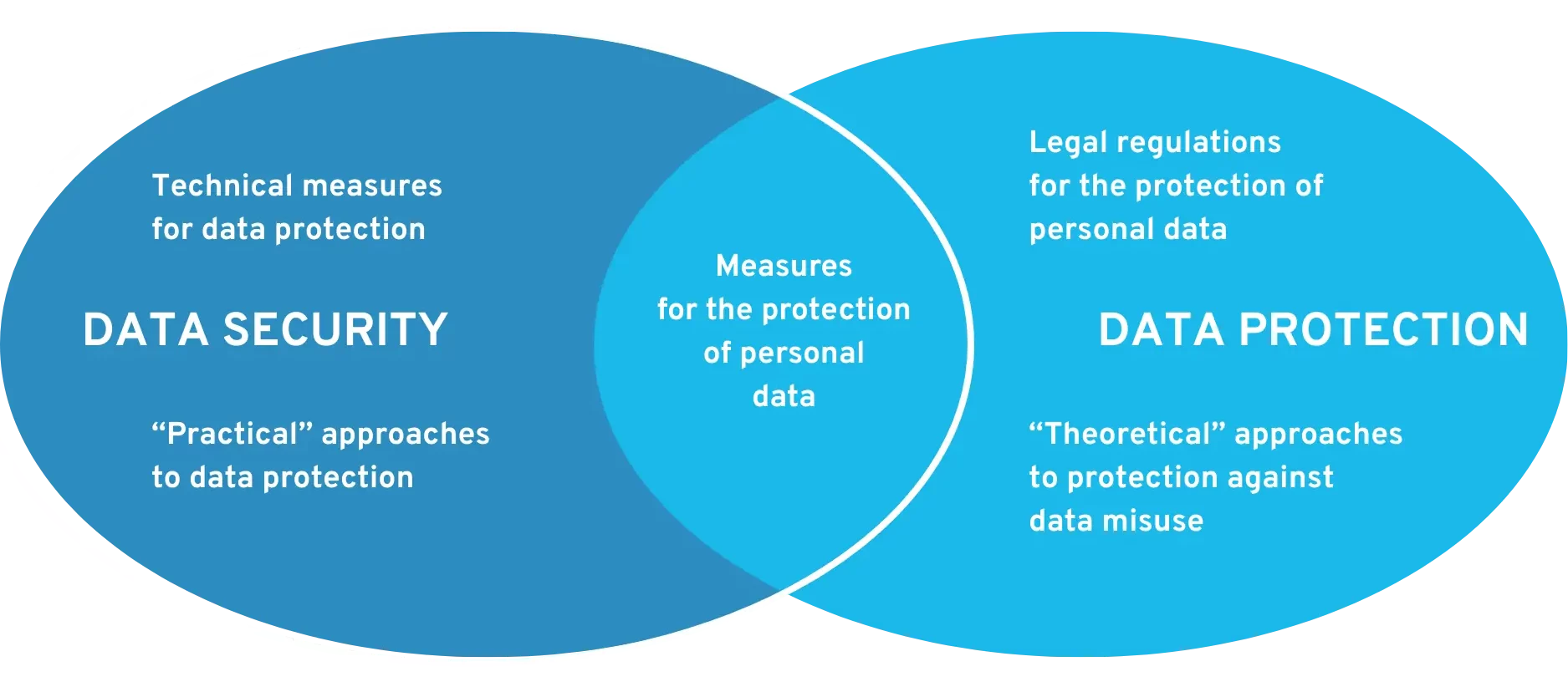 Requirements for data security and data protection at DiGA
Requirements for data security and data protection at DiGA
The Medical Devices Act and the GDPR already set out requirements for data security and data protection. The BfArM also uses a questionnaire to ask for specific details, as can be seen in the DiGAV. From 2022 at the latest, certification in accordance with the ISO 27000 series will even be required. From 2023, all DiGA (including those already listed) must have a data security certificate from the BSI and a data protection certificate. Find out more in this article: Data security and data protection certificates for DiGA
In addition to the criteria mentioned above, there are several other conditions that we will not explain in detail here. You can find a complete list of all criteria in our article on the topic “Is your app a DiGA?”
If the application meets all criteria and is accepted by the BfArM, statutory health insurance companies will reimburse the costs of the application for one year. During this period, the manufacturer must demonstrate whether its app improves patient care. At the end of the year, an evaluation is carried out to determine whether the application will remain in the DiGA directory or not. In principle, the BfArM also advises companies on inclusion in the DiGA directory (subject to a fee).
4. Conclusion
The Digital Care Act is an important step toward enabling continuous innovation in the healthcare sector in Germany in the future. The specific provisions are a long-overdue step toward advancing the digitization of the healthcare system.
This will not only facilitate the market launch of health apps, but also accelerate it. This will enable more companies to bring innovative digital products to market.
Of course, not every health app is useful. It is therefore important to question the benefits of each health application. Health insurance companies should not be expected to pay for applications that offer no added value for patient treatment.




