Summary
ORIKO®
ORIKO® is a digital health application (DiGA) for adults with Attention Deficit Hyperactivity Disorder (ADHD).
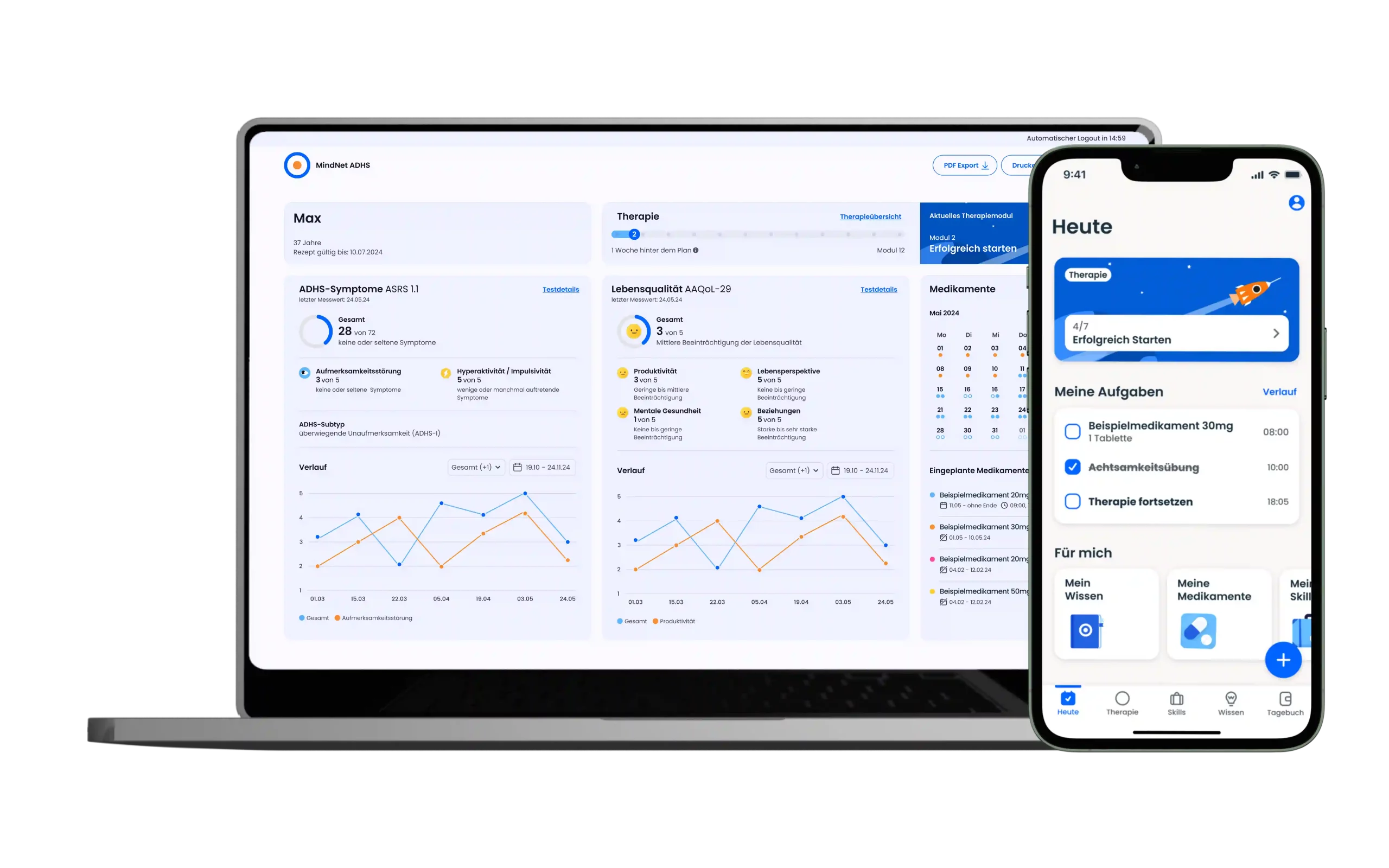
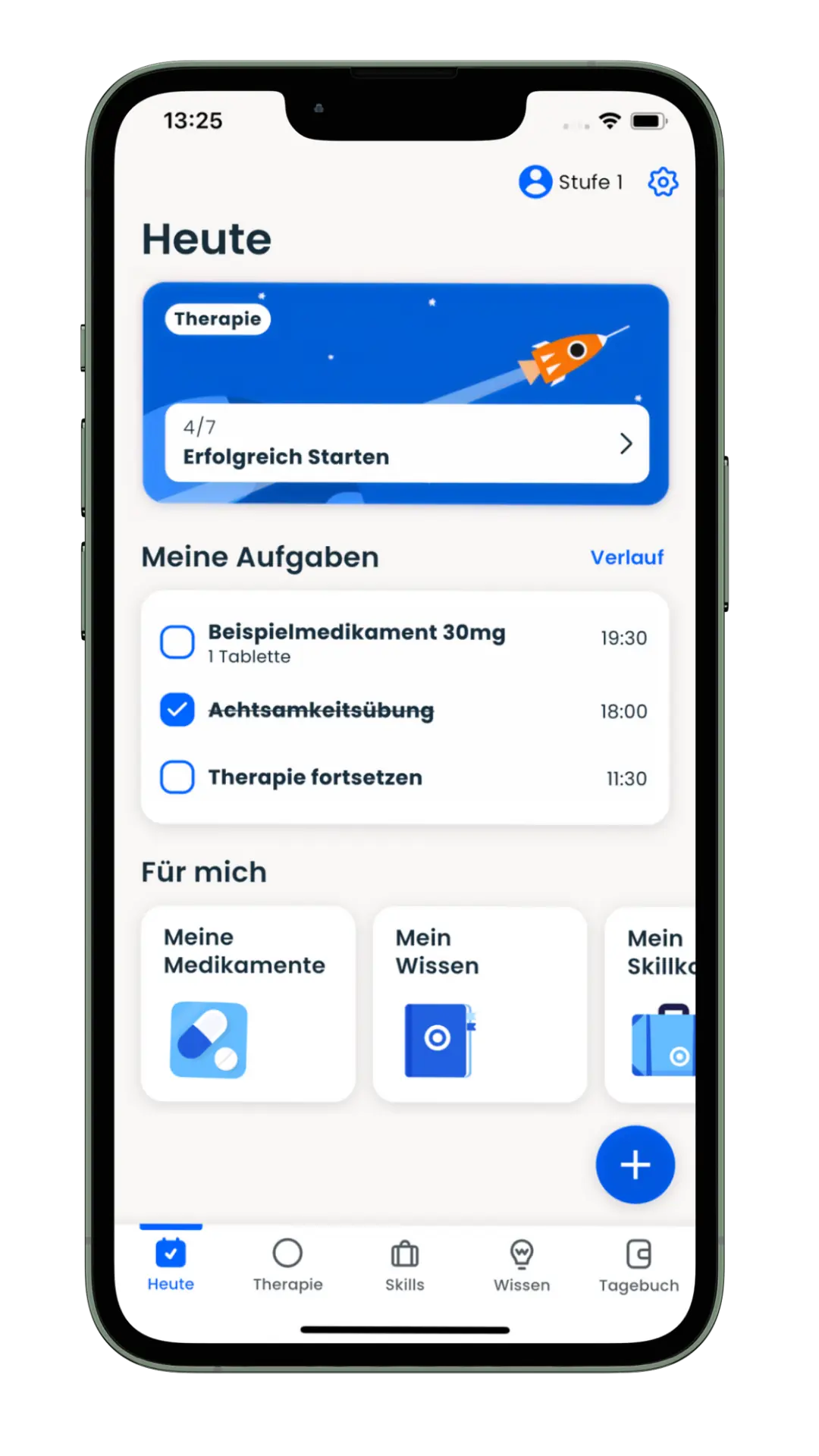
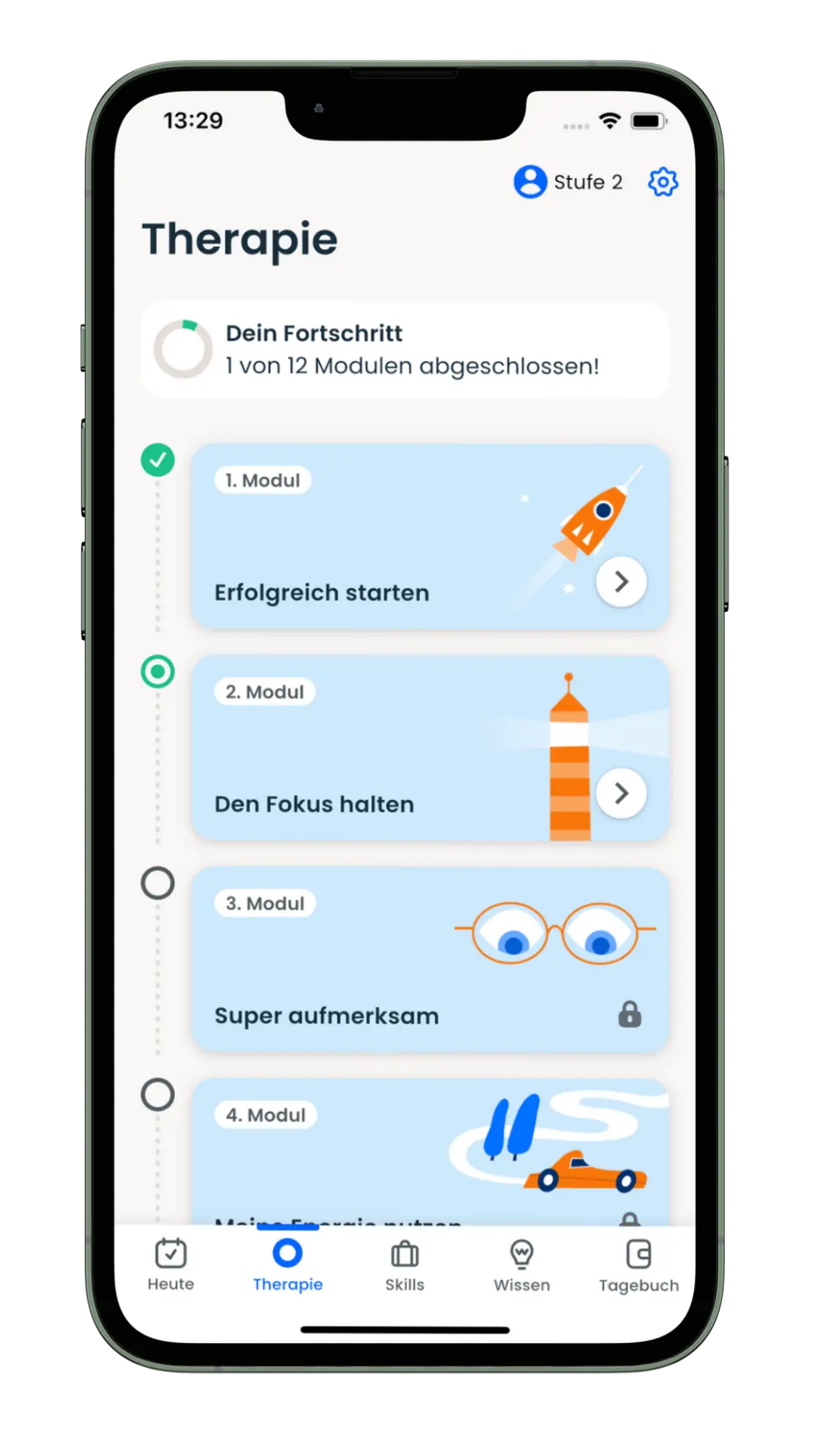
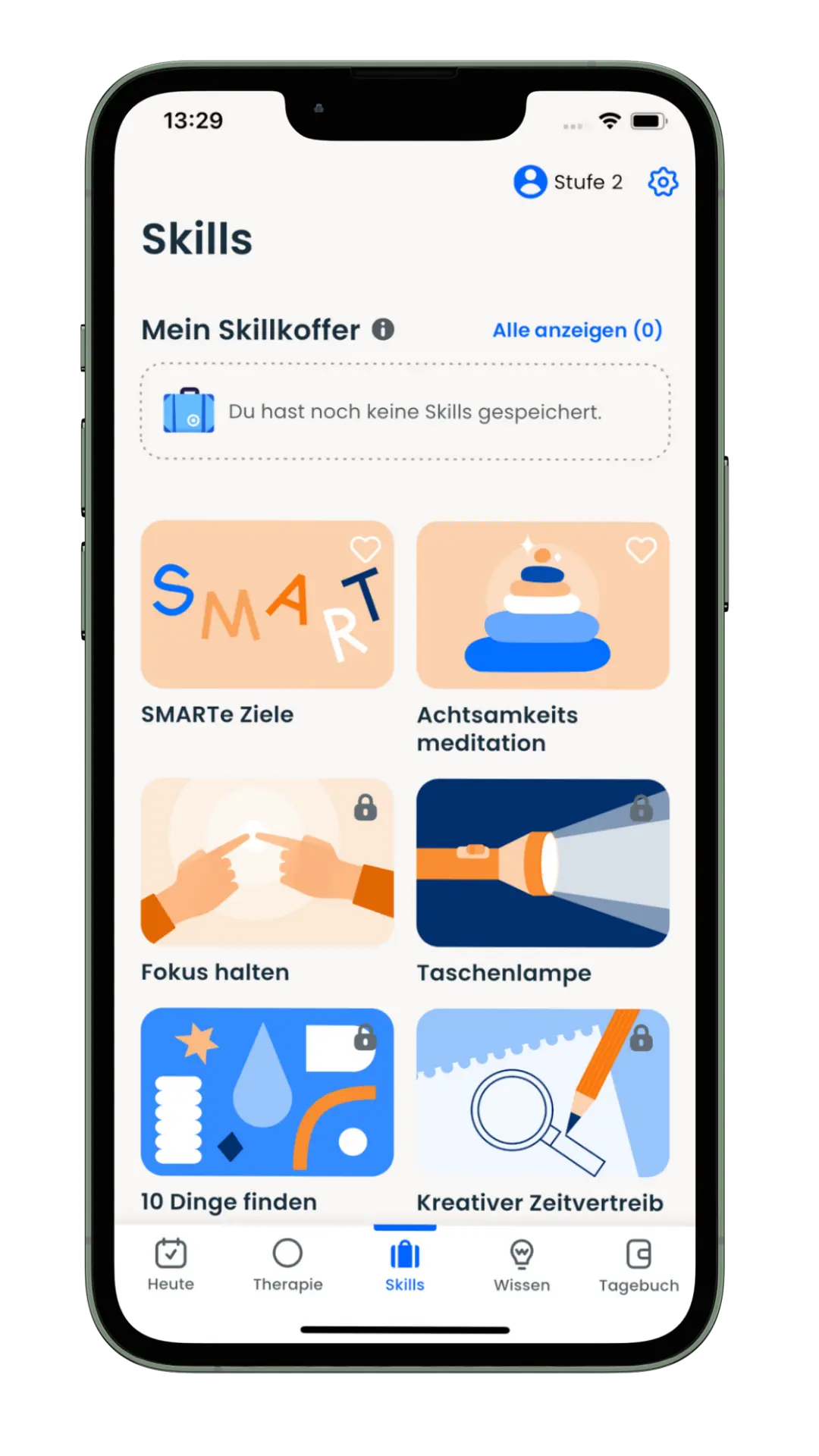
The app offers a 12-week, guided therapy program that provides medically sound knowledge and supports those affected in their everyday lives – evidence-based, digital and close to the needs of the target group. The app combines scientifically proven content with interactive tools such as diary functions, skills training and medication management. The product was developed together with psychiatrists, therapists and those affected.
ORIKO® has been listed in the BfArM’s DiGA directory since July 2025. The app has been clinically tested and with its inclusion in the BfArM’s DiGA directory, it can be prescribed by doctors and is reimbursable by statutory and most private health insurance companies.
Challenge
QuickBird Medical was responsible for the technical, regulatory-compliant development of . QuickBird Medical was also significantly involved in the content and conceptual design of .
In order to meet the requirements for a DiGA and be included in the DiGA directory, ORIKO® had to be developed as a medical device in accordance with the MDR and also fully meet the strict requirements of the BfArM. In addition to the regulatory obligations, the target group also posed a particular challenge: A digital solution for adults with ADHD must be low-threshold and motivating in order to encourage regular use in everyday life.
Achieving this balance of regulatory compliance, medical evidence, technical precision and user-centricity was the key to successful product development.
Process
ORIKO® was developed entirely within our ISO 13485-certified quality management system based on the IEC 62304 standard for medical software. Every development step, from architecture to verification, was fully documented. documented, tested and validated.
At the same time, the extensive requirements of the BfArM for DiGA had to be met in full: These included the technical connection to the telematics infrastructure (GesundheitsID, ePA) and the implementation of the extensive measures from BSI guideline TR-03161. In addition to the development within our certified information security management system in accordance with ISO 27001, ORIKO® also had to obtain an official security certificate in accordance with BSI TR-03161 in order to be listed as DiGA.
Result
ORIKO® is the first prescription-only DiGA for adults with ADHD. The DiGA has been listed in the official BfArM directory since July 2025.
Another huge success: ORIKO® is one of the first products on the entire DiGA market to receive an official BSI certificate for the new TR-03161 – proof of the extremely high standard of data security.
We would like to thank MiNDNET and Takeda for the trust they have placed in us and the excellent cooperation in the technical and regulatory implementation of their product vision.
Screenshots
Mobile
Desktop
Future
With the provisional inclusion in the DiGA directory, an important milestone has been reached, but the journey continues. The next goal: permanent listing as a reimbursable DiGA.
At the same time, the focus is now on reaching as many affected people as possible and giving them access to ORIKO®. We will continue to accompany this path – with technical developments, maintenance and regulatory expertise.