In 2020, Germany became the first country to establish DiGA in regular healthcare. This means that digital health applications can be approved in a structured, transparent process. Once approved, DiGA can then be prescribed by doctors in a similar way to medication. The costs are reimbursed by health insurance companies.
This process for approving DiGA served as a model for countries such as France and Belgium. Both countries developed similar approval and reimbursement processes. Austria also plans to integrate DiGA into its regular healthcare system.
Due to its location, it is also interesting to take a look at Switzerland. There, digital health applications are called “dGA.” Will the German DiGA model also be introduced in Switzerland in the next step? And what reimbursement options for dGA already exist there?
Update December 2025: Starting July 1, 2026, mandatory health insurance in Switzerland will cover the costs of digital health applications for cognitive behavioral therapy for mild to moderate depressive episodes, among other things. You can find more information on this in the specialist article below.
In this article, we focus on
- the fundamental characteristics of the Swiss healthcare system
- the question of whether dGA have a fixed definition in Switzerland
- the reimbursement options available to date and
- the question of whether a DiGA-like approval process will be established in Switzerland.
Table of Contents
- 1. Overview: Structure of the Swiss healthcare system and health insurance
- 2. Requirements for DiGA
- 3. Definition of DiGA in Switzerland
- 4. Current reimbursement options for DiGA
- 5. Future: DiGA Fast Track coming soon to Switzerland?
- 6. Conclusion
1. Structure of the healthcare system and health insurance
To better understand the environment in which DiGA operates in Switzerland, let’s first take a look at how the healthcare system works there. This is important because the healthcare systems in European countries have developed historically and are rarely comparable with one another: differences in structure, responsibilities, institutions, and financing systems ultimately create very different incentive systems for all groups involved.
Switzerland, as a country in Europe but outside the EU, is no exception here.
1.1 The healthcare system
The Swiss healthcare system is decentralized. While laws and regulations are set at the federal level, practical implementation is largely left to the cantons, which have a high degree of autonomy: A canton in Switzerland is a territory with its own government, parliament, and laws, which together with other cantons form Switzerland.
Health insurance is also organized at the cantonal level and by private companies. This results in a fragmented healthcare landscape, but one that generally offers the Swiss population very high-quality care in exchange for a high level of personal financial contribution.
While legislation is primarily at the federal level, the cantons have considerable freedom to shape implementation. This results in a fragmented healthcare landscape.
1.2 Health insurance
Since 1996, compulsory health insurance (OKP) has been mandatory in Switzerland. OKP covers healthcare costs once the annual minimum contribution has been exceeded. Insured persons continue to pay 10% of the costs up to a maximum of currently CHF 700 (adults) or CHF 350 (children) per year.
Premiums vary depending on place of residence, age, chosen deductible (franchise), additional benefits, and insurance model. They are set individually by around 40 insurers and approved annually by the Federal Office of Public Health. Employers do not contribute.
The Federal Office of Public Health determines which services are covered by this compulsory insurance for the whole of Switzerland. In addition, health insurance companies offer a wide range of supplementary insurance policies and special rates.
The high level of personal contributions from insured persons, rising costs, and growing premiums pose major challenges for the system.
2. Requirements for DiGA
In our article ‘Medical devices and Switzerland: what you need to know about MepV and MDR‘, you will find a detailed list of the requirements you must meet in order to market your software medical device in Switzerland. This does not affect the issue of reimbursement:
- Approval means that a medical device may be officially sold and used in Switzerland. Put simply, this requires the product to demonstrate that it is safe and fulfills its medical purpose. Approval is regulated by legal requirements. You can find more information on this in our guide to medical device approval in Switzerland.
- Reimbursement means that health insurance companies cover all or part of the cost of a product, so that patients do not have to pay (everything) themselves.
This article focuses primarily on the latter: all paths to reimbursement for digital health applications in Switzerland.
3. Definition von dGA: DiGA in der Schweiz
In November 2024, the Federal Office of Public Health defined DiGA as follows in its updated ‘Fact sheet on the reimbursement of digital health applications within the framework of OKP’:
Digital health applications (dGA) are products that promote health, support and inform patients, or serve medical purposes and use digital technologies.
A dGA can be software, an app, a mobile device (e.g., sensors for recording body parameters and software for digital evaluation or transmission), or a combination thereof. Artificial intelligence (AI) may also be used. A dGA can also be used in combination with a non-digital physical product (e.g., bathroom scales). dGAs can be intended for self-use by patients or healthy individuals or for use by healthcare professionals.
Switzerland uses a very broad definition for digital health applications, referred to here as ‘dGA’.
The following purposes for a dGA are therefore possible:
- Health promotion (e.g., fitness, nutrition, exercise, or wellness apps)
- Support and information (general, disease-related information and behavioral recommendations, with symptom tracking if applicable; if intended for healthcare professionals, medical use is also conceivable)
- Medical purpose (early detection, prevention, diagnosis, monitoring, prognosis, treatment or alleviation, as well as care of illnesses or their consequences, or in connection with maternity)
The following product types are included:
- Software products
- Products containing sensors
- Products that are used in conjunction with external hardware.
Only products intended for medical use are subject to the Medical Devices Ordinance (MepV).
You can find more information about the various reimbursement options for digital health applications in the next chapter.
4. Current reimbursement options for dGA
You are probably interested in whether DiGA can be reimbursed under the Swiss healthcare system and whether there is a special approval process like the German DiGA fast track.
Digital health applications with a medical purpose are treated in Switzerland like any other (non-digital) medical device. A discussion paper from 2022, which is still relevant today, outlines the following possibilities:
4.1 Remuneration within MiGel (list of means and items)
DiGA can be included by the Federal Office of Public Health (BAG) in the services covered by OKP (compulsory health insurance). This is the minimum level of care that health insurers must offer their insured persons. What this minimum level of care includes is determined at national level by the BAG and therefore applies in all cantons.
To this end, DiGA would have to be included in the ‘list of medical aids’ (MiGeL), a kind of list similar to that which exists in Germany. This list contains all medical aids that are covered by compulsory health insurance (OKP). Examples include wheelchairs and hearing aids. For each product, product groups, price caps, quantities, and requirements for cost coverage are listed.
The MIGEL directory in Switzerland is only partially suitable for digital health applications, as the processes it provides for mapping innovations are too lengthy. They often take several years and thus stand in stark contrast to the short innovation cycles of digital technologies.
In addition to complying with the rules set out in the MepV, medical devices must also meet the so-called EPE (WZW in German) principle in order to be included in the MiGeL. When an application for reimbursement under OKP is submitted, they are checked for
- Effectiveness
- Appropriateness
- Economic efficiency
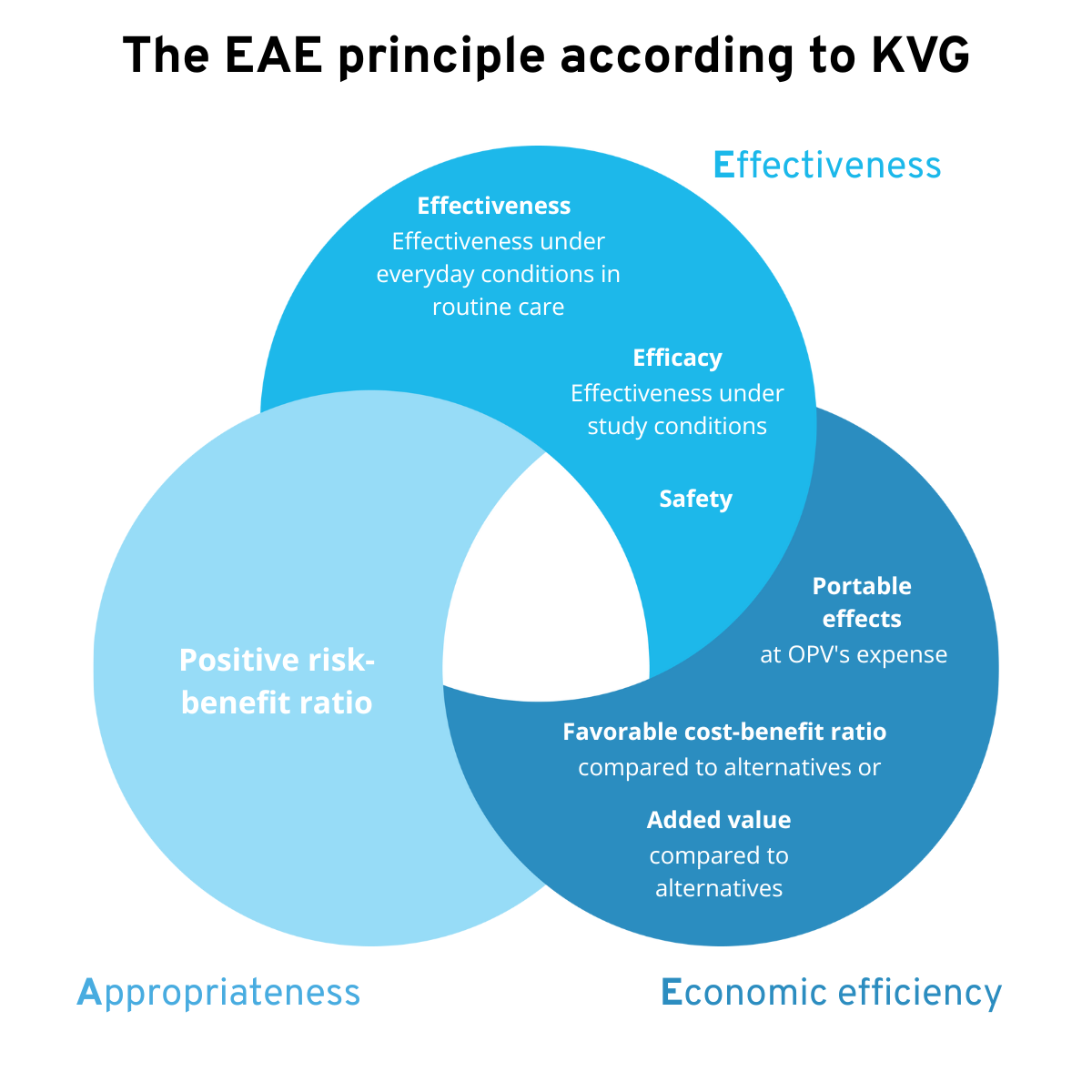
The EAE principle according to KVG
Source: Kohler, S., & Rau, A. (2023, April 26). Remuneration of digital health applications (DiGA) in Switzerland. VISCHER.
Current developments: The first positive developments in this area came at the end of 2025. Starting July 1, 2026, mandatory health insurance will cover the costs of DiGA for cognitive behavioral therapy for mild to moderate depressive episodes and recurrent depressive disorders, which are used as a supplement or bridge to psychotherapy. The first DiGA listed in the MiGeL is “deprexis,” which is already permanently listed in Germany.
4.2 Principle of trust and medical services
For DiGA that are related to medical services, the so-called trust principle applies. This principle states that medical services are generally assumed to meet the legal requirements for effectiveness, appropriateness, and cost-effectiveness.
However:
There is currently still some uncertainty regarding the specific application of this principle to digital applications. At present, there are also no billing models specifically geared toward digital services in the medical fee schedule. The integration of such services into existing medical fees has not yet been conclusively regulated either.
4.3 Flat-rate fees
Flat-rate fees have so far been used primarily in inpatient care, but are also planned for outpatient care. DiGA could be part of a comprehensive treatment program that is reimbursed at a flat rate. In such cases, it must be clearly defined which services are included in the flat rate and how these are taken into account in the tariff.
DiGA could be integrated in particular if they are part of a standardized treatment pathway that also includes medical prescriptions and specialist support for patients.
4.4 Bundled payments
Bundled payments combine various services from a treatment pathway across multiple service providers and remunerate them at a flat rate. They can be used in particular for planned procedures and the care of chronically ill patients. This is a relatively new option for billing services.
Treatment pathways are defined that encompass various service components (e.g., medical services, DiGA, accompanying care).
However, new remuneration models such as Bundled payments are struggling to gain acceptance because existing structures and a lack of incentives on the part of the social partners are standing in their way. Obstacles include insufficient involvement of service providers, dual hospital financing, the obligation to contract—i.e., the obligation of health insurance companies to conclude contracts with all approved service providers—as well as the ban on multi-year contracts and legal uncertainties.
4.5 Obligation to perform in evaluation
According to Art. 33, para. 3 KVG, the Federal Council may stipulate that new or controversial services – including digital services – are reimbursed under a temporary obligation to provide benefits under certain conditions, as long as their effectiveness, appropriateness, and cost-effectiveness have not yet been conclusively clarified. This procedure is referred to as ‘obligation to provide benefits under evaluation’. A federal checklist is used to assess whether a DiGA could be included in this procedure.
4.6 Pilot projects within the framework of the KVG
Under Article 59b of the draft KVG, pilot projects may be initiated to test new models for cost control, quality assurance, or digitization. These projects may be granted specific exemptions within the framework of the KVG, but they are limited in time and scope and are subject to evaluation.
Their implementation requires agreement between the relevant social partners, as well as substantial preliminary work by the project partners. The results of such pilot projects could serve as a basis for subsequent legislative changes, particularly in the development of new remuneration or care models.
4.7 Self-payers and supplementary health insurance
Finally, it should be mentioned that it is possible to simply launch a DiGA on the Swiss market as a medical device and market it individually to self-paying patients or enter into negotiations with approved private health insurers regarding cost coverage under private supplementary insurance plans. However, a guide published by the Digital Center Bülach (LinkedIn) points out that coverage by one or more health insurers excludes coverage via the channels described above. The reverse is also true.
5. Future: DiGA Fast Track coming soon to Switzerland?
The question now is whether Switzerland will take inspiration from its neighbors and establish a separate approval and reimbursement process for ‘dGA’, known as DiGA Fast Track.
However, a glance at the Swiss eHealth Strategy 2.0 reveals no mention of DiGA. ‘Digital applications’ are only mentioned in the context of electronic patient records. However, this does not appear to have been implemented in line with the legislator’s intentions, as the Federal Council has called for further development in summer 2023.
The follow-up program DigiSanté: Promoting Digital Transformation in Healthcare also makes no mention of plans to implement special approval and reimbursement procedures for DiGA. The central goal of the program is to ‘close Switzerland’s gap in the digitization of healthcare’.
Nevertheless, a cautious opening of the OKP to digital therapies is evident. The planned inclusion of digital applications for cognitive behavioral therapy in the scope of benefits from July 1, 2026, shows that digital health applications are increasingly being regarded as eligible for standard care, at least for specific indications. In the medium term, this could also promote discussion about more structured approval and reimbursement procedures for other digital applications.
6. Conclusion: reimbursement of dGA
In Switzerland, digital health applications (DiGA) are not yet integrated into compulsory health insurance as a separate category, and there are no special approval or reimbursement channels such as the DiGA fast track in Germany. Initial attempts to offer digital health applications through supplementary insurance or special programs are in place, but remain limited. However, with the latest developments in psychotherapy applications, MiGeL could establish itself as a promising reimbursement channel for DiGA manufacturers in Switzerland in the long term.
Our newsletter will keep you up to date on the status of DiGA in Switzerland and other countries.
For an overview of the reimbursement process for digital health applications in other EU countries, please refer to the following white papers and specialist articles:
- DiGA in Germany
- DiGA in France
- DiGA in Belgium
- DiGA in Austria
- DiGA in Italy
- Overview of DiGA approval in all EU countries
About us: At QuickBird Medical, we develop digital health applications and medical software on a contract basis for customers in the healthcare industry. We are certified according to ISO 13485 and ISO 27001. If you are planning a digital product in the healthcare sector, please feel free to contact us using the contact form.




