What began in Germany at the end of 2019 was taken up by neighboring France in 2023: The DiGA Fast Track now has a French counterpart in the form of “PECAN.“
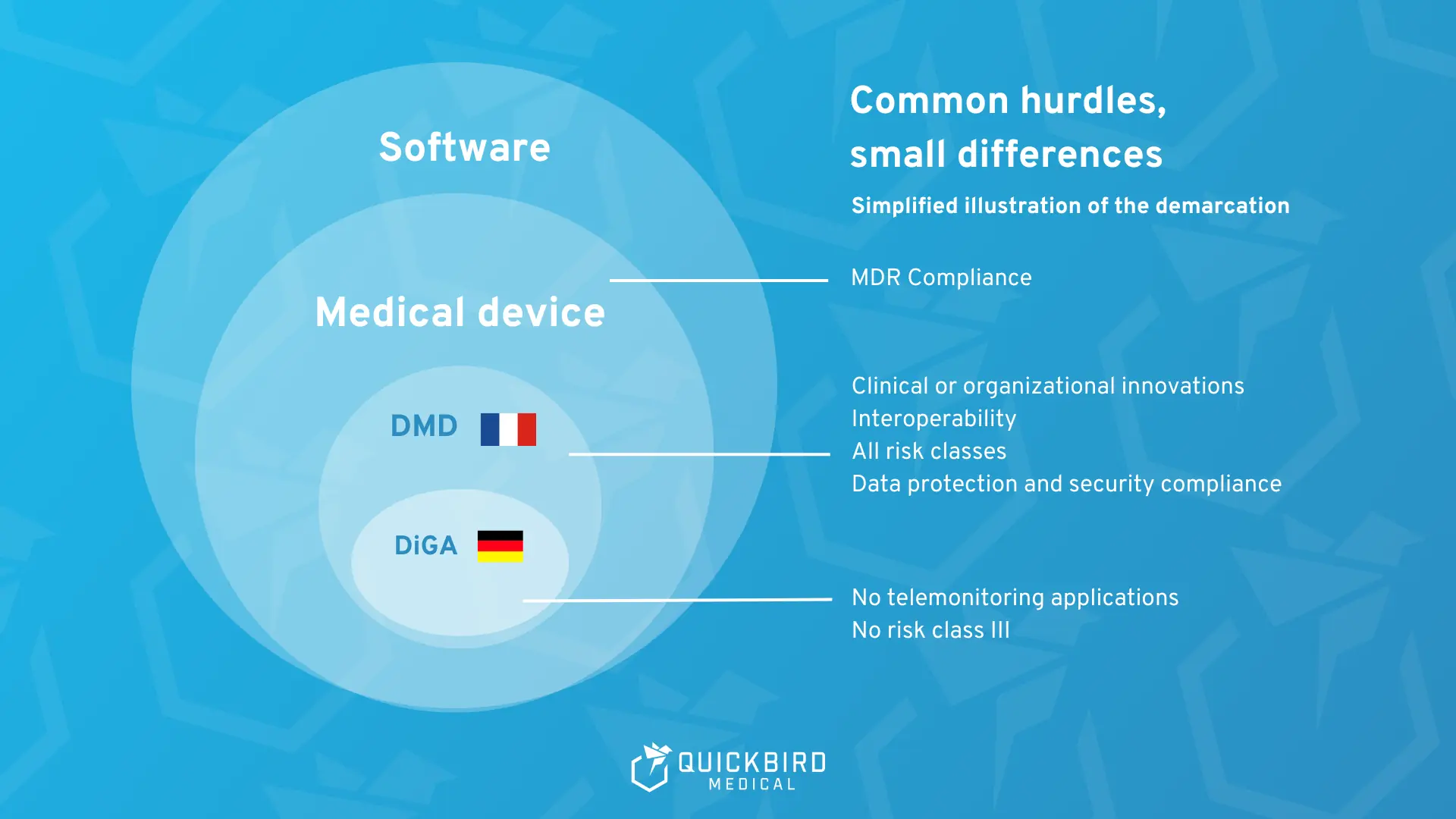
What is known as a “digital health application” (DiGA) in Germany is called “Dispositifs Médicaux Numériques” (DMN) in France and “Digital Medical Device” (DMD) in an international context.
This creates a structured fast-track procedure for manufacturers to have digital medical devices reimbursed by statutory health insurance in France for a limited period of time.
In this article, we provide a guide that explains …
- how PECAN works,
- how the procedure differs from the German reimbursement process,
- what requirements apply,
- and why transferring a DiGA to France may seem obvious, but is challenging in practice.
Table of Contents
- 1. Background: DiGA Model in Germany
- 2. Introduction: Reimbursement of DMD in France: PECAN
- 3. Regulatory Framework of PECAN
- 4. PECAN Application Procedure
- 5. Procedure following a positive PECAN Decision
- 6. Reimbursement of DMD costs
- 7. Clinical Evaluation in the PECAN Procedure
- 8. Stakeholders and Responsibilities
- 9. Status in 2026: France’s DiGA directory
- 10. Comparison: DiGA Fasttrack vs. DMD and PECAN
- 11. From Germany to France: DiGA to PECAN
- 12. Helpful Links
- 13. DiGA Models in other countries
- 14. Conclusion: The DMD framework as DiGA with additions
1. Background: DiGA Model in Germany
Since 2019, Germany has had a standardized reimbursement pathway for “digital health applications” (DiGA) in the form of the DiGA Fast Track. Manufacturers can apply to the Federal Institute for Drugs and Medical Devices (BfArM) for inclusion in the DiGA directory. As part of the fast track, the BfArM checks whether the application meets the regulatory and formal requirements (including MDR/CE, data protection/IT security) and whether a positive healthcare effect has been demonstrated. If the decision is positive, the DiGA is included in the directory and is then eligible for reimbursement by statutory health insurance funds.
The fast track offers two possible entry routes:
- Either permanent inclusion occurs immediately if proof of benefit is already complete,
- or provisional inclusion if a complete study on the positive effect on healthcare does not yet exist. In this case, the manufacturer must still submit an evaluation concept including evidence from a pilot study. If the evaluation is positive, the DiGA is then reimbursed, while the manufacturer submits the missing data for a complete study during the so-called trial year. Only after successful completion can the application remain permanently in the DiGA directory.
The French model is based on this German model for the reimbursement of digital health applications, but at the same time has some fundamental differences.
2. Introduction: Reimbursement of DMD in France: PECAN
PECAN stands for “Prise en Charge Anticipée Numérique.” Literally: provisional digital cost coverage. Since 2023, the procedure has enabled manufacturers to provisionally reimburse digital medical devices with presumed innovative character for a maximum of twelve months through statutory health insurance.
With PECAN, France has primarily adopted the idea of provisional reimbursement from the DiGA fast track. The key difference to Germany is that the transition to permanent reimbursement does not take place in the same procedure. Permanent reimbursement must then be applied for via a separate regular route (LPPR or LATM), which existed long before PECAN.
Furthermore, digital health applications are not referred to as DiGA in France, but as “Dispositifs Médicaux Numériques” (DMN) and, in an international context, as “Digital Medical Devices,” or DMD for short
3. Regulatory Framework of PECAN
The legal basis for the PECAN procedure is Article L. 162-1-23 of the French Social Security Code (Code de la Sécurité Sociale, CSS). This means that PECAN is enshrined in law and regulated as a provisional reimbursement mechanism that is legally binding.
In addition, the French health authority Haute Autorité de Santé (HAS) provides guidelines that serve as a practical handbook for manufacturers. It not only describes the scientific requirements for presumed benefit at , but also specifies the formal criteria for technical evidence, particularly with regard to interoperability and IT security. The document also contains current practical examples. Compared to the German DiGA guidelines, it is significantly more compact at around 27 pages.
3.1 Scope
The scope covers two categories of digital medical devices:
- digital medical devices with therapeutic purposes and
- digital medical devices for medical remote monitoring.
In comparison, the scope of application of the German DiGA Fast Track is narrower and does not include telemonitoring applications.
3.2 Key Principles
According to the guidelines, the PECAN procedure follows four principles that manufacturers should be aware of:
- Fast track to provisional reimbursement:
PECAN is a separate procedure that precedes regular reimbursement. - Strictly limited in time:
Provisional reimbursement is valid for a maximum of 12 months from the date of the decision; an extension is not possible. Further reimbursement is only possible via a separate application for standard care (LPPR or LATM). - Cumulative eligibility criteria:
All legal requirements must be met simultaneously - Technical certification:
In addition to the assessment by the Commission nationale d’évaluation des dispositifs médicaux et des technologies de santé (CNEDiMTS), certification by the Agence du Numérique en Santé (ANS) is required. The individual institutions are examined in more detail in chapter 8.
3.3 Reimbursement Criteria and Requirements for DMD in France
In order for a DMD to be eligible for the PECAN procedure, the following criteria and requirements must be met. It is important to note that the assessment is always indication-specific. A product may therefore be PECAN-eligible for one indication but not for another.
3.3.1 Reimbursement Criterion 1: CE Marking according to MDR
In short: no CE, no PECAN.
As in Germany, not all software is a digital health application, but every DMD is a medical device and subject to the Medical Device Regulation (MDR).
For PECAN, this means:
- The DMD must have a valid CE marking according to MDR
- The indication applied for must correspond to the indication for CE certification according to MDR.
This is how it is checked:
- The indication in the instructions for use is decisive.
- If the indication stated in the PECAN application differs in terms of wording or content, the manufacturer must clearly justify and explain this in the dossier.
Further information on the approval and certification of medical device software according to MDR can be found in our guide on the subject.
3.3.2 Reimbursement Criterion 2: Presumed Innovation
In addition to the CE marking, the DMD must be considered presumed innovative. The decisive factor here is whether the technology:
- can be expected to have clinical benefits
or - a measurable improvement in the organization of care
Two minimum requirements always apply:
- Organizational progress must not compromise the quality of care.
- Ongoing studies must be available for the digital medical device that are expected to provide sufficient data to subsequently apply for permanent reimbursement.
Germany uses a similar assessment approach for this purpose: Instead of “presumed innovation,” the DiGA fast track focuses on the “positive effect on care,” which must be demonstrated either through medical benefit (mN) or through patient-relevant structural and procedural improvement (pSVV).
3.3.3 Reimbursement Criterion 3: Technical Requirements
Parallel to the evaluation by the CNEDiMTS, the ANS checks the technical requirements.
Specifically, the DMD must:
- comply with data protection requirements,
- comply with national interoperability and IT security standards,
- enable standardized, interoperable data export,
- offer interfaces to devices for recording vital parameters.
An official interoperability certificate is also required for permanent reimbursement, including in conjunction with the French national eHealth ID (INS).
Data protection, information security, and interoperability are also key requirements for the DiGA fast track in Germany.
Reimbursement criteria for PECAN compared to DiGA
3.4 Reasons for Exclusion
Provisional reimbursement is not possible if:
- the DMD already had a PECAN reimbursement for the same indication,
- a PECAN has already been rejected
→ Exception: New data in cases of previously insufficient evidence, - there is an official suspension or ban,
- PECAN is to be combined with other reimbursement mechanisms (e.g., LPPR, LATM, innovation flat rate, transitional reimbursement).
4. PECAN Application Procedure
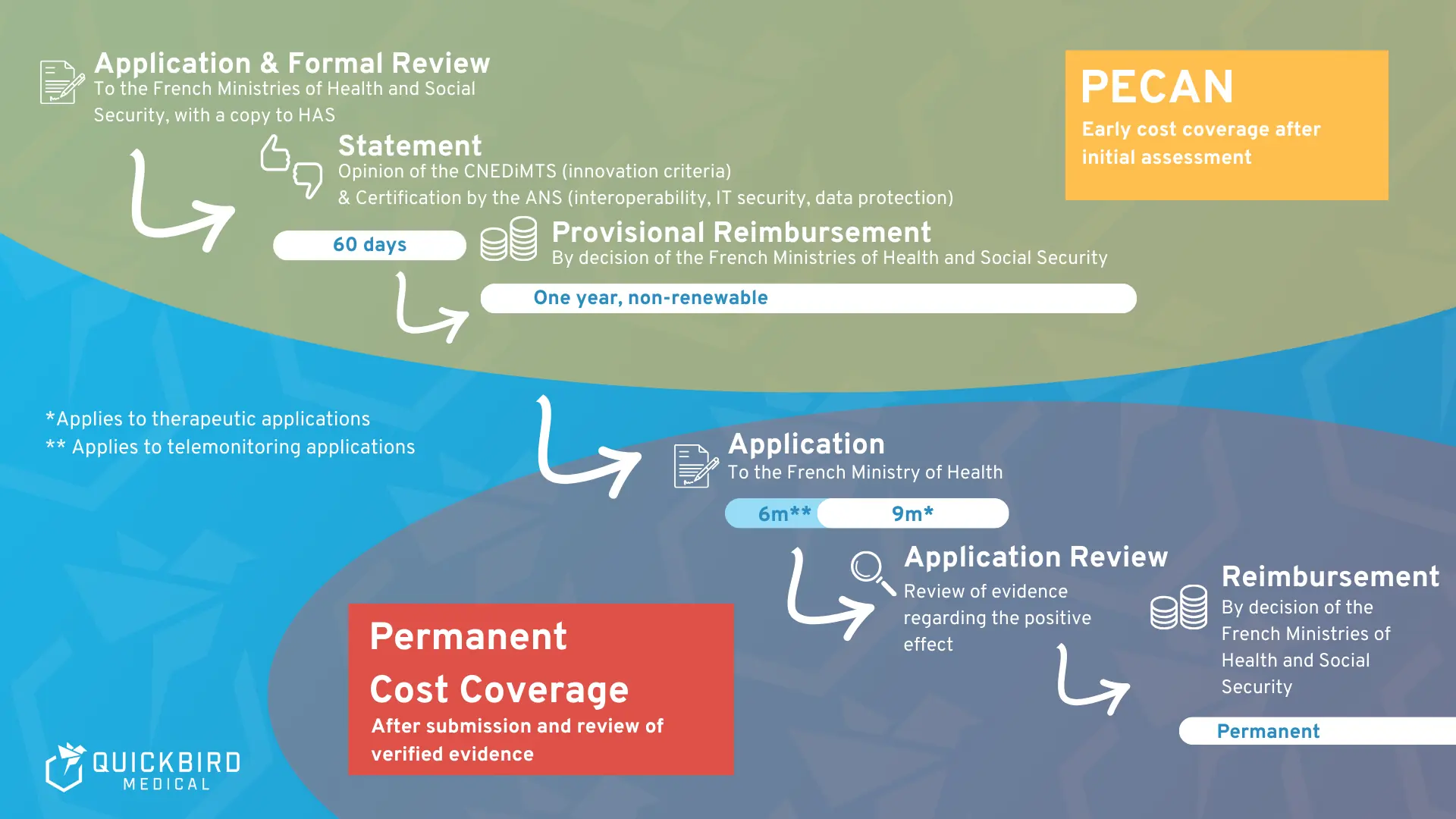
The application procedure consists of three phases in total.
- Submission & formal review of the dossier
The manufacturer submits the dossier to the relevant ministries of health and social security, with a copy to the HAS.
In this first phase, the following is checked:
- Is the dossier complete?
- Does it meet the formal requirements of the HAS?
If any documents are missing, the manufacturer will receive a request for additional information.
Deadline: 30 days to submit the missing information. If this deadline is missed, the application is classified as “withdrawn.”
- Opinion by the CNEDiMTS
Once the dossier is complete, the CNEDiMTS will assess its eligibility. The focus is on the indication and presumed innovation.
The assessment is carried out without consulting or hearing the applicants.
Processing time: maximum 60 days from receipt of the complete application.
The result is a positive or negative statement, which is sent to the ministries.
- to the ministries,
- the manufacturer,
- and the relevant expert committees (CNP) and is published on the HAS website.
- Decision on provisional reimbursement
The relevant ministries make their decision based on the CNEDiMTS opinion.
Deadline: 30 days
The decision is published in the Journal officiel by ministerial decree and specifies:
- whether the product will be provisionally reimbursed,
- and under what financial conditions.
5. Procedure following a positive PECAN Cecision
After a positive PECAN decision, the digital medical device will be provisionally reimbursed for a maximum of twelve months. During this time, the manufacturer must prepare for the transition to permanent reimbursement. PECAN serves as a bridge to regular reimbursement:
- For digital medical devices with therapeutic purposes, the regular reimbursement route is inclusion in the list of reimbursable products and services (LPPR). The manufacturer must submit the follow-up application within 6 months of the PECAN decision. The HAS also provides guidelines for this.
- For digital medical devices for remote medical monitoring, the regular reimbursement route is inclusion in the list of remote medical monitoring activities (LATM). The manufacturer must submit the follow-up application within 9 months of the PECAN decision. The HAS also provides guidelines for this.
The figure below summarizes the PECAN procedure and the subsequent regular reimbursement channels.
PECAN procedure and subsequent reimbursement route for standard care
6. Reimbursement of DMD Costs
Here we discuss the specific regulations governing reimbursement of DMD costs within the framework of PECAN and thereafter.
6.1 Reimbursement amount during the PECAN Year
During the PECAN procedure, prices are regulated by government-defined flat rates. These include:
Digital therapeutics (DTx)
- An initial package costing €435. This can be billed once per patient. It applies to the effective use of the digital medical device for a maximum of 3 months.
- A follow-up package that follows on from the initial package. This is billed on a pro rata basis according to the intended prescription period and actual use and amounts to €38.30 per month.
- The maximum financial compensation, consisting of the two aforementioned lump sums, is €780 per patient per year.
Further information can be found in paragraph II of Article R. 162-117 of the Social Security Code.
For telemonitoring solutions
- the technical operator receives a monthly flat rate of €50 to €91.67, depending on the proven interest.
- The medical monitoring service is reimbursed separately (e.g., by a doctor or nurse).
Further information can be found in Article L162-52 of the Social Security Code.
6.2 Reimbursement after the PECAN Year has expired
After the PECAN year, the flat-rate reimbursement ends. For permanent reimbursement, the price is then renegotiated in the respective regulatory pathway via LPPR (for DTx) or LATM (for telemonitoring applications), based on usage and with the involvement of the Comité économique des produits de santé (CEPS).
LPPR for digital health applications or DTx
In the LPPR regulatory pathway, reimbursement is based on a price per unit. It is important to note that the actual product price may be higher than the amount reimbursed by the health insurance fund. The difference is then covered by the patient, their supplementary health insurance (Mutuelle) or another organization.
LATM for telemonitoring
In the standard LATM pathway for telemonitoring, two flat-rate amounts are reimbursed per patient:
- a flat rate paid to the telemonitoring operator, and
- a flat rate paid to the operator.
The rates for the technical packages are determined taking into account the organizational or clinical interest that can be expected from remote medical monitoring. Clinical interest is assessed based on the impact on quality of life, morbidity, or mortality.
7. Clinical Assessment in the PECAN Procedure
Clinical assessment is a central component of the PECAN procedure. The CNEDiMTS examines whether a DMD can be considered innovative and whether the studies submitted and ongoing are suitable for creating the basis for regular reimbursement via LPPR or LATM in the short term. The principles of evidence-based medicine are decisive in this regard.
7.1 Evidence-based and context-dependent
The assessment is not carried out in isolation from healthcare practice. The CNEDiMTS always assesses the clinical relevance of the data in the context of:
- the underlying disease,
- its epidemiology,
- and the therapeutic strategy in which the digital medical device is embedded.
Another decisive factor is whether the results can be transferred to the French healthcare system. In cases where recruitment opportunities are limited, international multicenter studies may be accepted. However, this is subject to the condition that their transferability to the French system can be comprehensibly justified.
7.2 Data Basis
In principle, the CNEDiMTS prefers to rely on product-specific clinical data. In certain cases, however, non-product-specific data may also be taken into account, for example on technologically comparable solutions or earlier versions of the product.
In these cases, the following conditions must be met:
- a conclusive justification of technical equivalence,
- a transparent analysis of possible differences,
- and proof that these differences have no relevant influence on the clinical or technical effect.
7.3 Clinical Benefit and Endpoints
Clinical benefit is always assessed in comparison with a relevant alternative. In doing so, the CNEDiMTS takes into account both the positive effects and the potential risks of use.
Examples of evaluation dimensions in the CNEDiMTS PECAN guideline are:
- Quality of life,
- morbidity or mortality,
- Complications,
- Reduction in unplanned hospital stays.
It is important that the selected endpoints are appropriate for the claimed indication and the promised effect of the product. In practice, it has been shown that endpoints that are formally correct but inappropriate in terms of content often lead to critical evaluations.
7.4 Organizational Benefits as an argument for Innovation
In addition to clinical benefits, advances in the organization of care can also be used to justify the adoption of innovations. This can be achieved, for example, through new care processes or improved accessibility of care, particularly in the area of remote monitoring.
However, the CNEDiMTS notes that these benefits are often only described in applications, but not systematically substantiated. It is therefore recommended that organizational effects be presented in a structured manner, e.g., based on the actors involved, changed processes, and appropriate evaluation criteria.
8. Stakeholders and Responsibilities
The following actors in the French healthcare system are particularly relevant to the PECAN procedure:
- CNEDiMTS (Commission nationale d’évaluation des dispositifs médicaux et des technologies de santé or National Commission for the Evaluation of Medical Devices and Health Technologies): Evaluates medical and care-related evidence.
- ANS (Agence du Numérique en Santé, or National Agency for Digital Health): Reviews technical requirements such as data protection, interoperability, and IT security.
- Ministère chargé de la Santé & Ministère chargé de la Sécurité sociale (Ministries of Health and Social Security): Make the final decision on provisional cost coverage and implement it by ministerial decree, based on the opinions of the CNEDiMTS and the ANS.
- HAS (Haute Autorité de Santé, French Health Authority): Offers, among other things, so-called “early consultation meetings.” These are aimed at manufacturers whose products are still in clinical development. The content of these meetings can include discussions on clinical evidence strategy, optionally in combination with health economic topics (efficiency assessment). Important: The consultations are optional, non-binding, confidential, and free of charge. They are not considered an assessment and do not allow any conclusions to be drawn about the subsequent decision of the CNEDiMTS.
- G_NIUS (“Guichet National de l’Innovation et des Usages en e-Santé” or National Contact Point for Innovation and Applications in E-Health): This is a national platform for promoting innovation in the field of digital health. It supports developers and manufacturers in particular in navigating the regulatory environment, identifying financing and funding opportunities, and networking with relevant players in the French e-health ecosystem. The aim is to facilitate and accelerate the market launch of new digital technologies.
9. Status in 2026: France’s DiGA Cirectory
Although the PECAN procedure has been operationally available since 2023, it has so far played only a minor role in healthcare practice. Unlike in Germany, there is no independent public DMD register in . Digital medical devices only appear indirectly in existing reimbursement structures:
- LPPR (Liste des Produits et Prestations Remboursables): central reimbursement directory for all medical devices, without labeling digital applications
- LATM (Liste des Activités de Télésurveillance Médicale): directory for reimbursable telemonitoring services, also without explicit DMD status
This means that there is no public overview of which DMDs are currently (provisionally) reimbursed.
So far, only one application that has actually been reimbursed via PECAN is publicly verifiable:
Cureety TechCare
- Telemonitoring solution for oncology patients
- Inclusion in PECAN: 2023
- No transition to permanent reimbursement via LATM
- The main reasons cited are a lack of or insufficiently reliable evidence of long-term clinical benefits and organizational added value.
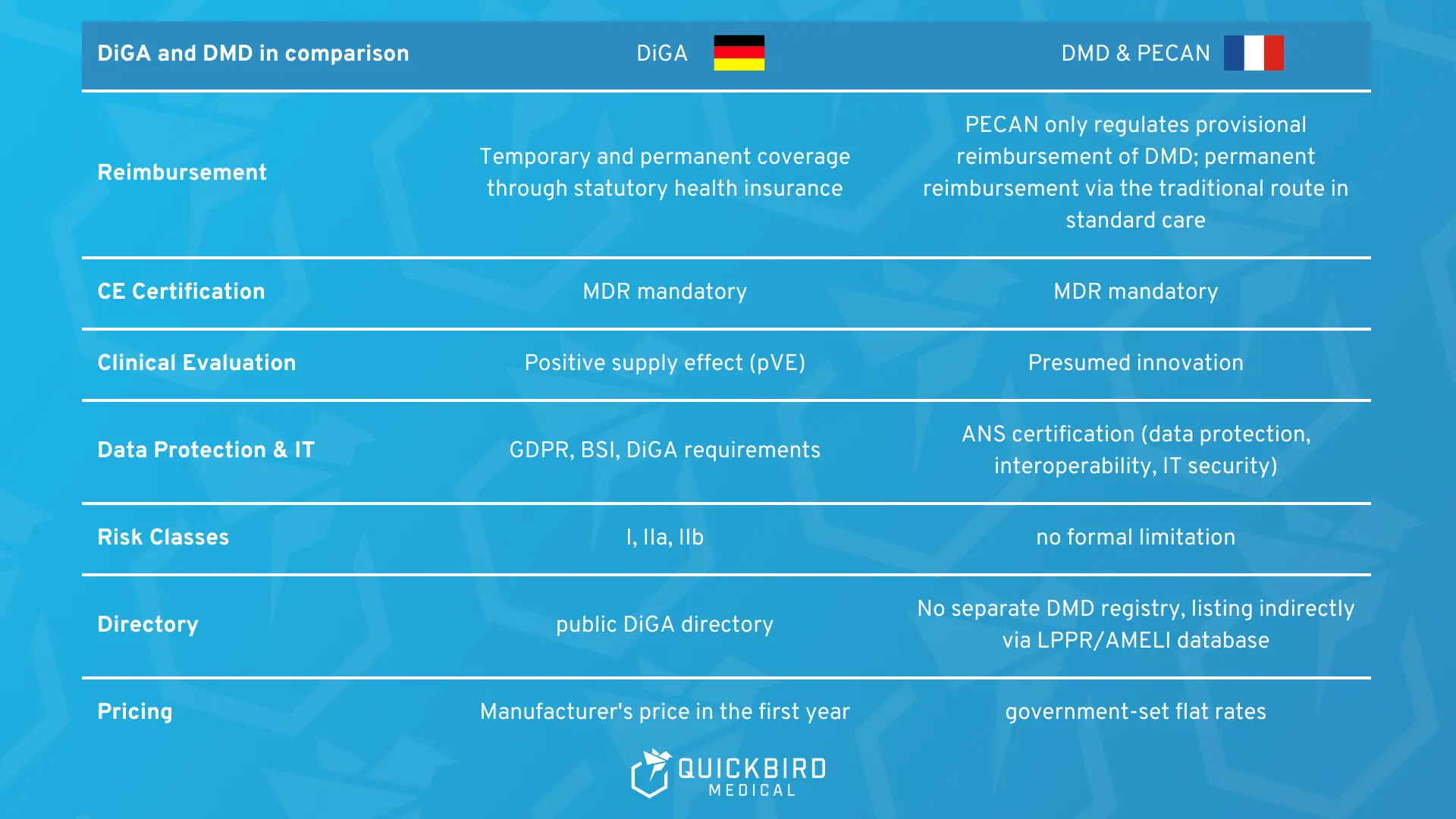
10. Comparison: DiGA Fasttrack vs. DMD and PECAN
Germany and France are pursuing the same goal: to bring digital medical devices into healthcare more quickly. The difference lies primarily in structure, transparency, and pricing logic.
- Reimbursement logic:
Both the German DiGA procedure and the French system provide for provisional reimbursement. In Germany, provisional and permanent reimbursement are anchored within the same DiGA regulatory framework. In France, PECAN reimbursement is explicitly designed as a temporary entry point and is limited to twelve months. Permanent reimbursement subsequently requires a separate application via LPPR or LATM, i.e., established reimbursement mechanisms that are not specifically tailored to digital applications. - Reimbursement:
In both countries, statutory health insurance funds cover the costs of reimbursement. - CE certification:
In both systems, CE marking according to MDR is mandatory. - Clinical evaluation:
Both procedures are evidence-based. DiGA focuses on the positive effect on care, while PECAN focuses on presumed innovation plus robust study design. - Data protection & IT security:
The requirements are similarly high in both countries. Germany bundles the assessment in the DiGA procedure. France separates it organizationally: clinically via the CNEDiMTS, technically via the ANS. - Risk classes:
In Germany, the DiGA fast track is limited to medical devices in risk classes I, IIa, and IIb. Class III products are not included.
PECAN does not contain any formal limitation of the MDR risk class. - Register & transparency:
In Germany, the DiGA directory is a separate, public register exclusively for DiGA applications. A comparable, separate directory for digital medical devices does not yet exist in France, which makes it much more difficult to obtain a transparent overview of the market. - Pricing:
In the DiGA fast track, manufacturers are free to set the price during the trial period, provided they comply with the maximum amounts. For permanent listing, they must negotiate a reimbursement amount with the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds).
PECAN, on the other hand, works with government-fixed flat rates in the preliminary phase. The final price is negotiated with the CEPS after the PECAN expires as part of the regular reimbursement process.
Differences between DMD and DiGA Fast Track
11. From Germany to France: DiGA to PECAN
After comparing the two, the obvious question arises: if a DiGA works in Germany, why not in France?
On paper, there are many parallels: MDR basis, early reimbursement, focus on evidence. So can German DiGA manufacturers simply go to France with their existing products and studies to tap into this new market?
In practice, unfortunately, the transfer does not seem to be so easy. Evidence from clinical studies in Germany may not be so easily accepted in France.
11.1 Example: DiGA from “HelloBetter” – Application for PECAN Reimbursement
This is also illustrated by the HelloBetter Insomnia case. As a manufacturer of several DiGA listed in Germany, HelloBetter also submitted a dossier in the PECAN procedure.
The result was a negative opinion from the HAS and thus no provisional reimbursement via PECAN. The reason given was that the data submitted did not sufficiently substantiate the required “presumption of innovation.” Thus, sufficient clinical benefit or progress in care had not been demonstrated.
The case makes it clear that DiGA evidence from Germany is not automatically PECAN-eligible. Differences in indication delimitation, comparators, care logic, and endpoints play a central role.
Statements:
- Statements from the HAS can be found here and here
- Statements from the manufacturer “HelloBetter” on this rejection can be found here
11.2 Looking ahead: German-French Rapprochement
Despite such setbacks, the direction is clear: harmonization instead of isolated solutions.
The G-BA and the HAS are already working on closer coordination, particularly with regard to:
- clinical evidence requirements
- methodological standards
- technical evaluation of digital medical devices
Goal:
Manufacturers should be able to use their evidence for both markets in parallel in the future.
12. Helpful Links
For further information on the reimbursement of digital health applications in France, here is a collection of important links:
Links relating to the PECAN procedure:
- Legal basis PECAN (CSS Art. L. 162-1-23): The legal framework for the provisional reimbursement of DMD.
- PECAN guide CNEDiMTS (HAS): Key document for manufacturers: evaluation logic, requirements, examples, typical reasons for rejection.
- Arrêtés on PECAN reimbursement (DTx, telemonitoring): The legal acts that define or update the flat rates/parameters for provisional reimbursement.
- PECAN overview (G_NIUS): Brief, easy-to-understand summary of PECAN, including classification in the reimbursement logic.
Links for reimbursement in standard care (LPPR & LATM):
- HAS “Principes d’évaluation / DM pathway” (2010 Guide): Overview of stakeholders and reimbursement pathways for medical devices in France (especially in the MDR context/market surveillance).
- LPPR/AMELI database: Public reimbursement environment for medical devices and telemonitoring services, without its own DMD register.
- LPPR guide (HAS): Official guide to applying for and evaluating the inclusion of medical devices in the LPPR.
- LPPR financing (G_NIUS): Overview of how reimbursement and pricing logic work in practice within the LPPR.
- LATM Guide (HAS): Official guide to submitting a dossier for telemonitoring services in the LATM pathway.
- LATM financing (G_NIUS): Explanation of the reimbursement logic in telemonitoring, including roles, flat rates, and billing principles.
13. DiGA Models in other Countries
More and more countries are planning a standardized process to bring digital health applications into standard care. In addition to France, you should also take a look at the following countries:
- Belgium (link to the guide for the approval of DiGA in Belgium)
- Austria (DiGA in Austria: Approval of digital health applications (2026))
- Germany (Insider tips for the approval of DiGA in Germany)
- Switzerland (Reimbursement of digital health applications (DiGA & dGA))
- Italy (Are DiGA coming to Italy soon?)
- Reimbursement of DiGA in the EU (Overview of DiGA approval in all EU countries)
14. Conclusion: The DMD Framework as DiGA with Additions
Since 2023, PECAN has been a clearly defined procedure that, for the first time, provides France with a structured path for the provisional reimbursement of digital medical devices.
At the same time, experience to date shows that PECAN is not yet a reliable route to market access. Transparency is limited, there is no separate register, and there seem to be hardly any publicly verifiable success stories. In addition, the transition to permanent reimbursement remains a high hurdle, especially if clinical benefits or organizational added value cannot be proven in a short time frame and in a manner specific to the indication. Even successful DiGA evidence from Germany cannot be automatically transferred to PECAN, as the case of HelloBetter shows.
For manufacturers, this means that PECAN is currently more of a challenging transitional instrument than a stable reimbursement channel. Anyone seriously pursuing PECAN should ensure that they have a robust study plan and a strategy that is geared towards permanent reimbursement under PECAN from the outset.
Are you looking to launch a digital application on the French market?
If you are planning to obtain approval for a digital application in France or Germany, please feel free to contact us. QuickBird Medical specializes in the contract-based development and approval of digital health applications and software medical devices. We develop your application, obtain approval for it as a medical device in accordance with MDR, and guide you step by step through the PECAN process.