Since 2020, there have been increasing efforts in Austria to integrate digital health applications (DiGA) into regular healthcare. So is the “app on prescription” now coming to Austria?
In this article we give you an overview of:
- the planned approval process in Austria
- the current status (2026) of the requirements for DiGA in Austria
- the timetable for the introduction of a DiGA approval process in Austria
Content of this article
- 1. Background of the DiGA concept
- 2. Initial situation
- 3. Approval as a medical device vs. reimbursement by health insurance companies
- 4. The path to reimbursement for DiGA in Austria
- 5. Previous reimbursement options in Austria
- 6. DiGA models in other countries
- 7. Conclusion
1. Background of the DiGA concept
In 2020, Germany pioneered the concept of a digital health application (“DiGA”). DiGAs are also known as “apps on prescription” and can be prescribed to patients by their doctor. A special approval process has been created for them in Germany: the DiGA fast track. Digital products that have successfully passed this approval process are reimbursed by health insurance companies on prescription.
Countries such as France and Belgium followed suit with their own approval and reimbursement processes, which are based on the German fast-track procedure.
In the future, the concept of a DiGA could also come to Austria – more on this in the chapters below.
2. Initial situation for digital health applications
Triggered by Germany’s introduction of the DiGA fast track, the discussion on the introduction of an approval process for digital health applications (DiGA) also intensified in Austria in 2020.
The Digital Austria Act has formed the basis of these discussions since June 2023. It aims to open up the Austrian healthcare system to digital innovations and create a legal framework for the integration of DiGA.
Under point 7.4 the following is listed:
“The prescription of quality-assured digital health applications should be made possible in cooperation with social insurance and supplement telemedical care.”
The exact form this approval process will take has not yet been conclusively clarified. Due to the intensive exchange with German stakeholders, it seems possible that Austria will work towards similar standards such as those in Germany. This is intended to ensure the quality and effectiveness of the reimbursable DiGA.
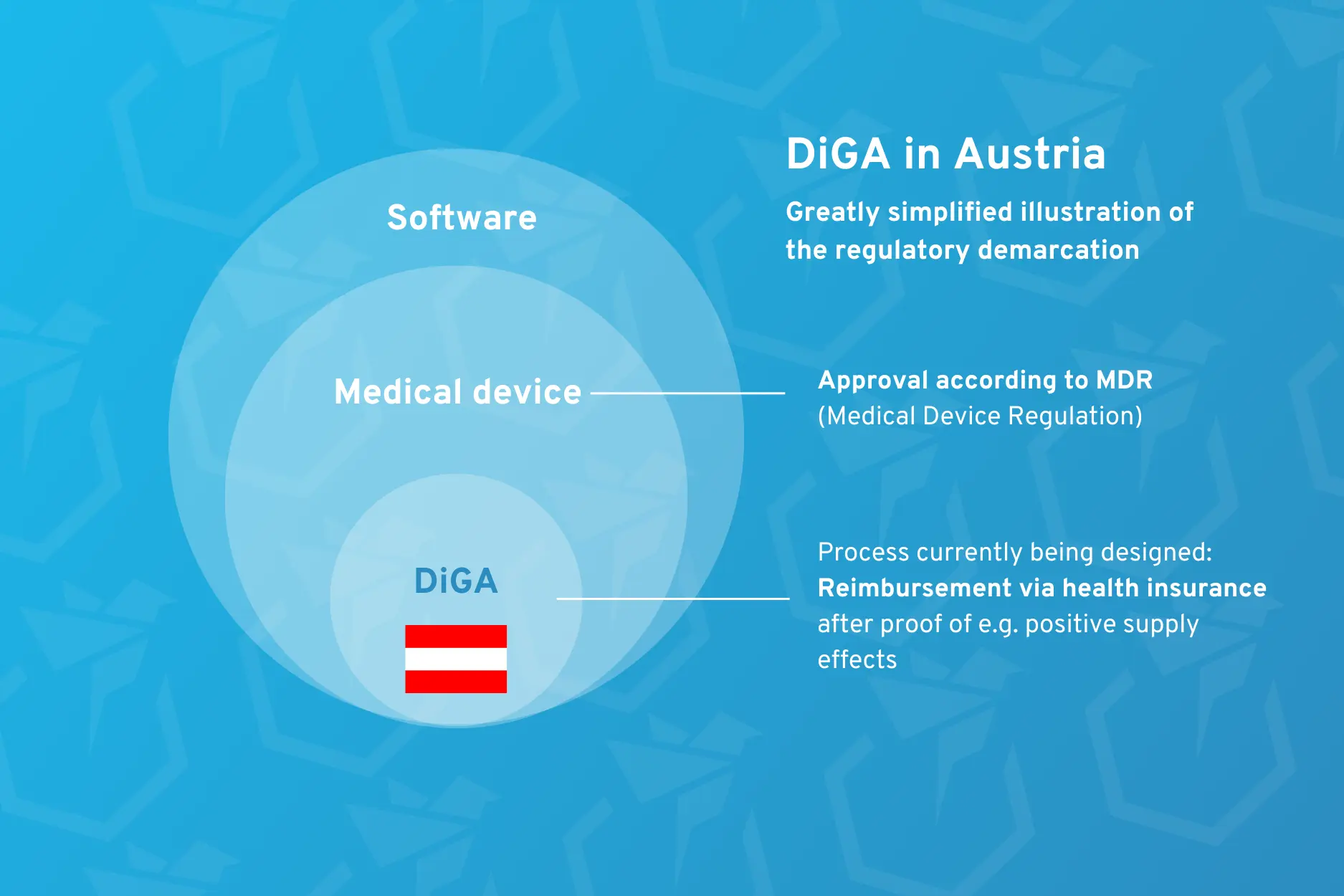
3. Approval as a medical device vs. reimbursement by health insurance companies
In the context of digital health applications in Austria, it is important to distinguish between two approval processes:
- The approval of (digital) medical devices in accordance with the Medical Device Regulation:
With MDR approval, you are authorized to place your medical device on the European market. However, this does not mean that you will also receive money from the health insurance companies for your product. For the time being, it is only a matter of approving that your product is safe and efficient and can therefore be used by users. - The approval of digital health applications (DiGA) for reimbursement by health insurance companies:
If you want to use the funding from health insurance companies to finance your digital product, you will have to deal with country-specific regulations. In Germany, for example, this is the DiGA approval process at the BfArM, which enables your product to be reimbursed by health insurance companies. Incidentally, your product must already be approved as a medical device in accordance with the MDR.
Bringing a digital medical device onto the market in Austria is currently already firmly regulated by the MDR and possible without any problems. However, a standardized process for how you can have a digital health product in particular reimbursed by health insurance companies in Austria is still being developed.
4. The path to reimbursement for DiGA in Austria
At present, the exact structure of the approval process is still largely unclear. Both manufacturers and official bodies such as the Austrian Agency for Health and Food Safety (AGES) often cite the German DiGA Fast Track as a reference. However, no individual aspects of the German procedure are emphasized. The possibility of provisional approval without completed proof of efficacy has also not yet been conclusively clarified.
However, there are some indications that provide more insight into the planned requirements of an Austrian DiGA approval process.
4.1 Requirements for digital health applications in Austria
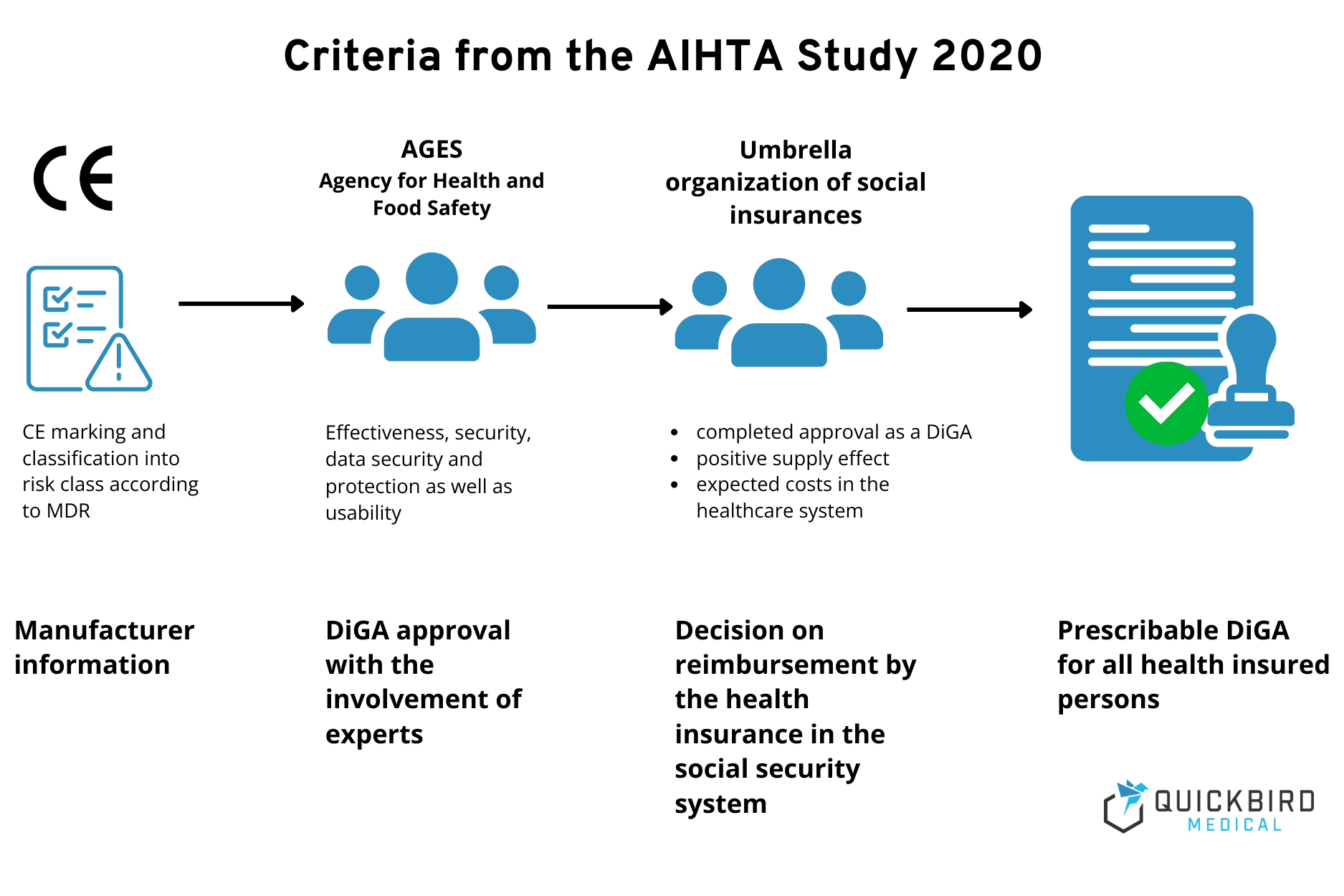
The AIHTA (Austrian Institute for Health Technology Assessment) published specific recommendations for DiGA requirements in the Austrian healthcare system in an initial study in 2020. The AIHTA is an independent institute for scientific decision support in the healthcare sector. The main shareholders are the Austrian federal states and the umbrella organization of the social insurance funds.
The AIHTA recommended the following criteria for the quality review of DiGA:
- In a first step, DiGAs are to be checked for the presence of a CE marking and classification according to risk classes in accordance with the MDR.
- The reported risk class according to MDR should then be used to determine the necessary evidence, such as the performance of studies. The AGES (Agency for Health and Food Safety) is responsible for this.
- Approval as a reimbursable DiGA is to be issued with the involvement of the umbrella organization of social insurance companies. The criteria are clinical efficacy and safety, expected costs in the healthcare system, data security and protection as well as usability and interoperability.
- According to the AIHTA’s recommendation, the process should be piloted and adapted in the process.
Many of the AIHTA’s criteria were also discussed at the LISAvienna Regulatory Conference in fall 2023:
- Adherence to therapy as a quality criterion
- Use of (anonymized) health data for medical professionals, manufacturers or for research purposes
- No breaks in the digital care pathway: connection to other digital solutions in the healthcare sector such as the Austrian patient file ELGA
- Need to publicize digital health applications in order to establish interest in DiGA on the part of patients and doctors
It is not yet clear which criteria will ultimately be adopted in the final approval process.
4.2 Timetable for DiGA in Austria
The requirements set by the umbrella organization of social insurance carriers and the Austrian Health Insurance Fund for DiGA are crucial for the regular reimbursement of DiGA. Only then will it be possible to predict which types of digital health applications will be eligible for reimbursement. The framework conditions developed during the pilot project in 2024, which we will present in the next subchapter, should provide some insight into this.
In June 2024, the Federal Ministry of Social Affairs, Health, Care, and Consumer Protection published the “eHealth Strategy Austria.” This sets out the specific measures for designing an approval process for digital health applications:
“M1.7: Creation of a uniform process for the evaluation of digital health applications (DiGAs) and digital care applications (DiPAs) and, subsequently, their introduction into healthcare if demonstrable benefits are identified. Prioritization/time frame: Short term (2024-2026).”
As of November 2025, the pilot process has now been completed. The legal framework is to be established by the end of 2026 so that DiGAs can also be prescribed in Austria from the beginning of 2027.
4.3 The piloting process for creating an Austrian DiGA process
The approval and reimbursement procedure for DiGA was tested in Austria as part of a pilot project by the Social Insurance Association. The practicality of the procedure developed was to be assessed on the basis of the evaluation of specific digital health applications.
The project was outlined in a written response to the SVDGV as follows:
| Project initiator | Umbrella organization for social insurance |
| Aim of the pilot project | Practical testing of evaluation criteria developed by the umbrella organization for social insurance |
| Duration | August 2024 to the end of February 2025, followed by the start of the implementation phase |
| Criteria for DiGA to be included in the procedure | – DiGA is a Class I or IIa medical device – Participation is subject to confidentiality – Willingness of the DiGA manufacturer to participate in meetings and answer questions – No remuneration for participation – No guarantee of actual subsequent remuneration for participating DiGA |
| Definition of project success | Participating DiGA should have gone through the previously developed HTA framework. This should have enabled an evaluation to be carried out. The developed procedure will then be presented. |
| Stakeholders involved | e-Health representative of the “Bundeskurie niedergelassene Ärzte” (Federal Association of Practicing Physicians) No involvement of patients or physicians. |
| Advantages for DiGA manufacturers through participation | Insight into the planned HTA process |
5. Previous reimbursement options in Austria
Digital health applications can already be reimbursed in Austria by way of exception. These applications fall under the category “Other necessary medical aids” in accordance with § 137 of the General Social Insurance Act (ASVG). Patients can therefore claim reimbursement under certain conditions.
According to § 133 Para. 2 ASVG, it is crucial that the DiGA fulfill the intended purpose without exceeding what is necessary. This condition is checked individually for each application, which means that reimbursement is only granted in individual cases.
The use of health apps has been promoted in Austria for some time now. A survey conducted by the Austrian umbrella organization for social insurance in 2018 revealed that 9 out of 16 organizations within the Austrian social insurance system provided a total of 22 health apps free of charge to their policyholders. It was striking that the majority of these offers did not require proof of an insurance relationship, which increases accessibility. In 2018, just over half of health insurers in Austria offered such digital health applications.
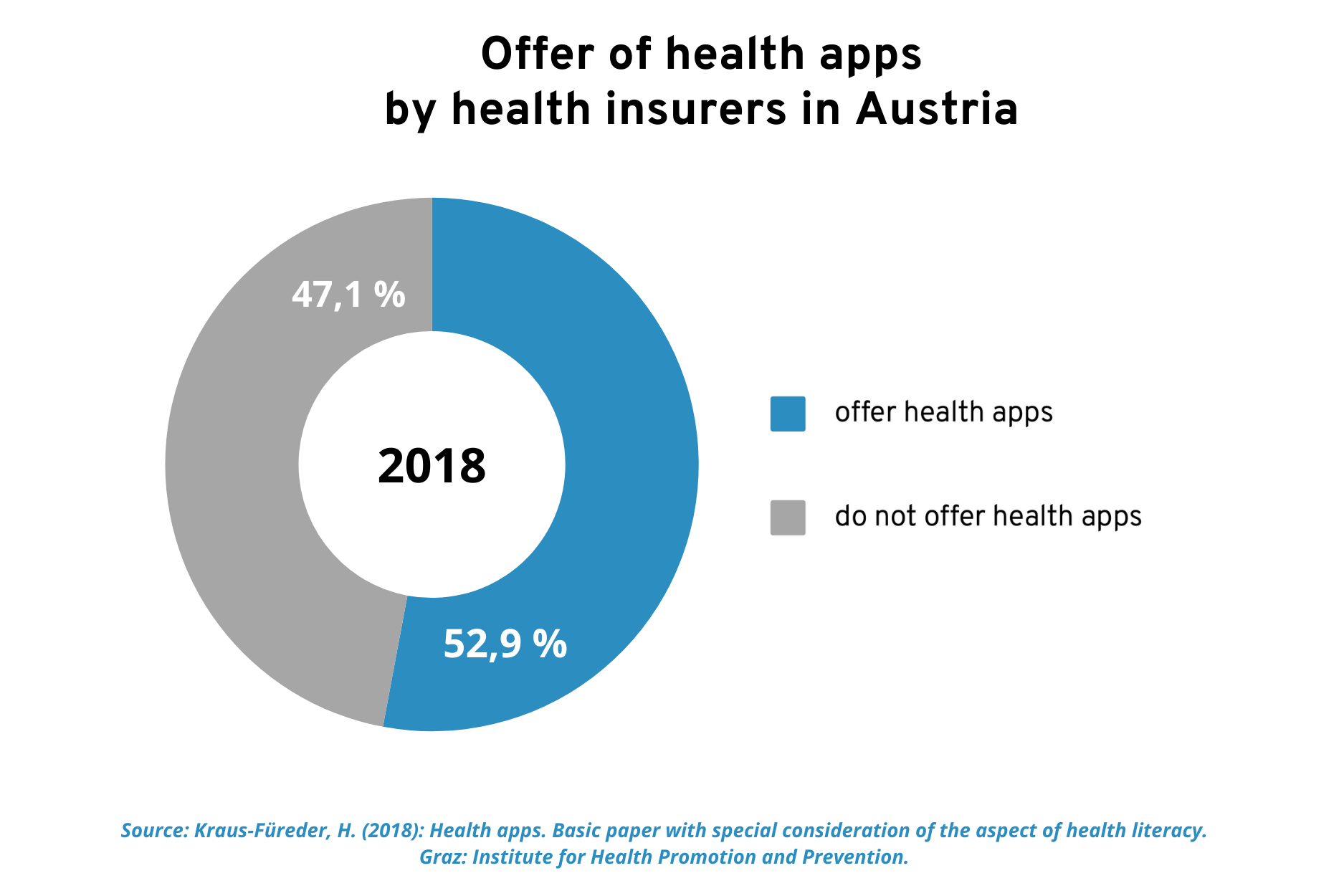
In 2018, just over half of health insurers in Austria offered their policyholders digital health applications.
A key issue in this context is the quality testing of the apps on offer. Until now, classification as a medical device was not a necessary criterion for the provision of apps. There is no standardized quality control for this. Nevertheless, an attempt was made to take certain basic quality criteria into account, as can be seen from the corresponding charts and evaluations.
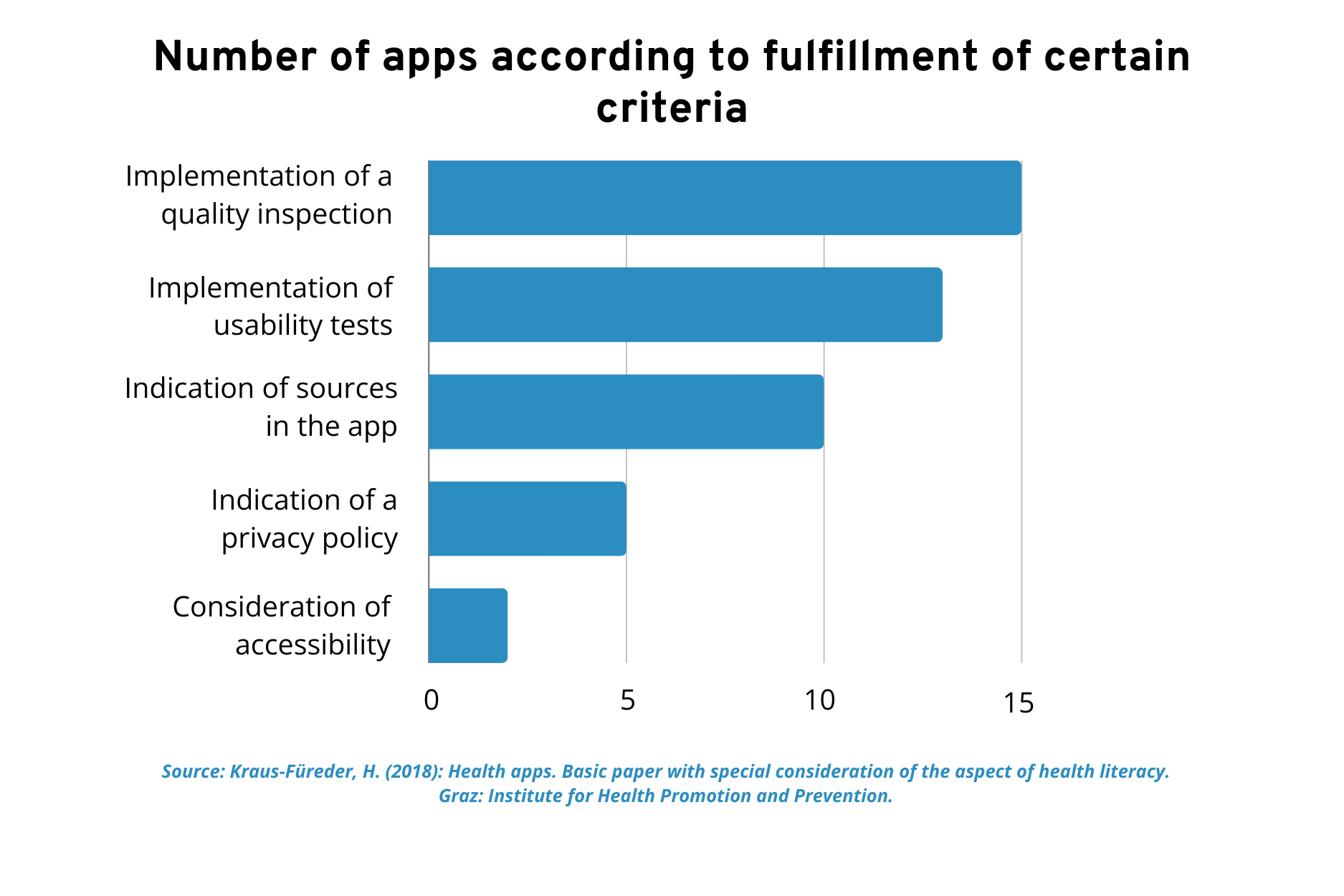
Classification as a medical device has not yet been a criterion in the development of health apps in Austria.
Interestingly, the majority of available applications focused on prevention. Only 5 of the 22 apps examined were integrated into a treatment or telemonitoring concept and thus specifically designed for people with the disease.
6. DiGA models in other countries
More and more countries are planning a standardized process to bring digital health applications into standard care. In addition to Austria, you should also take a look at the following countries:
- Belgium (link to the guidelines for the approval of DiGA in Belgium)
- France (link to the guidelines for the approval of DiGA in France)
- Germany (insider tips for the approval of DiGA in Germany)
7. Conclusion
In 2025, the Austrian discussion about the introduction of digital health applications (DiGA) seems to have gained momentum. Despite the great motivation expressed by all those involved, there is currently no legal approval process in sight.
The results of the AIHTA piloting process are eagerly awaited. The next important step is to define the requirements that a DiGA must fulfill in Austria. The specific requirements for reimbursement by the health insurance companies are then important.
Only then will there be a secure framework for manufacturers to sell DiGA in Austria.
We will update this article as new developments occur.




